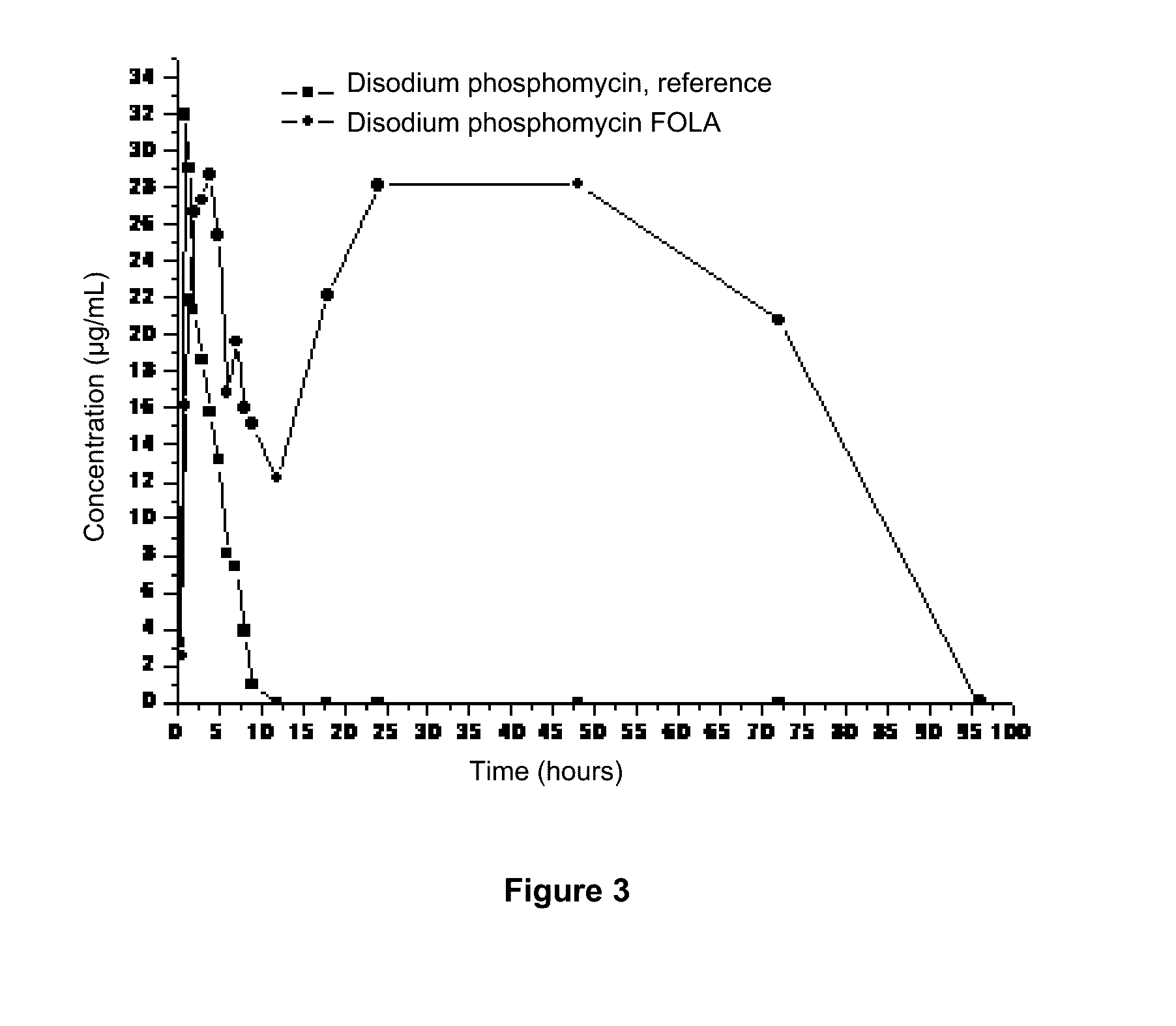
Patents
Literature
74 results about "Dosing drugs" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
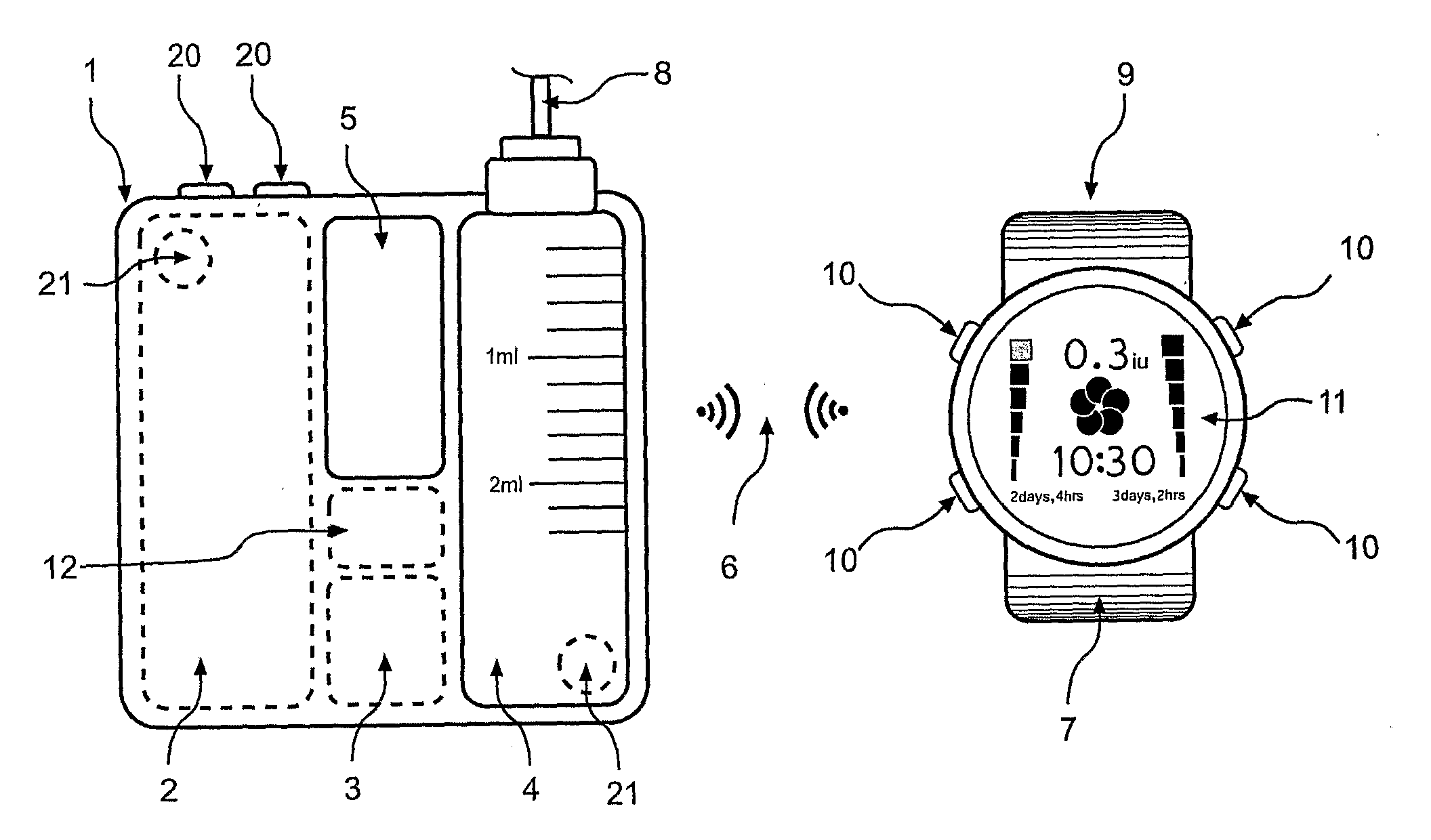
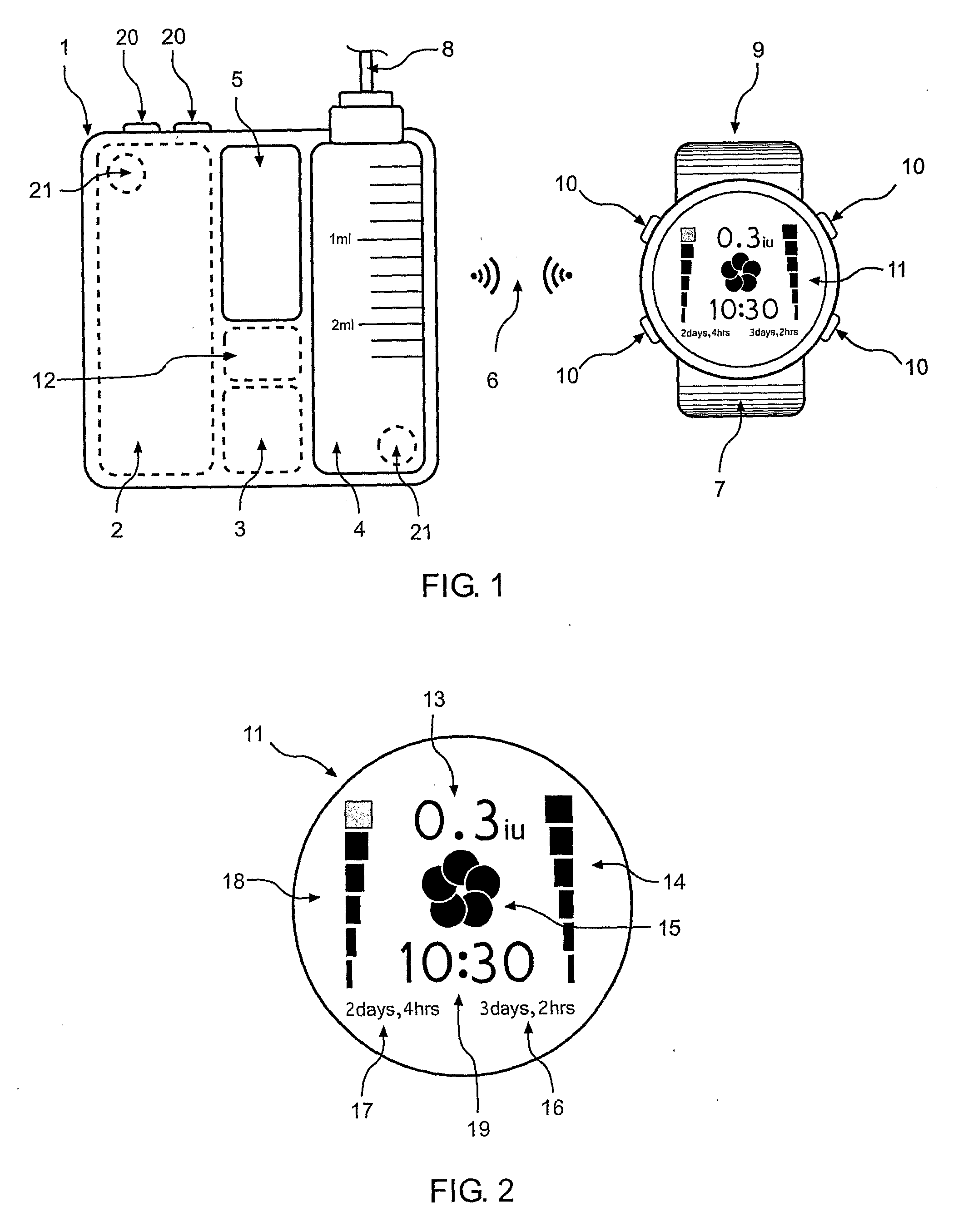
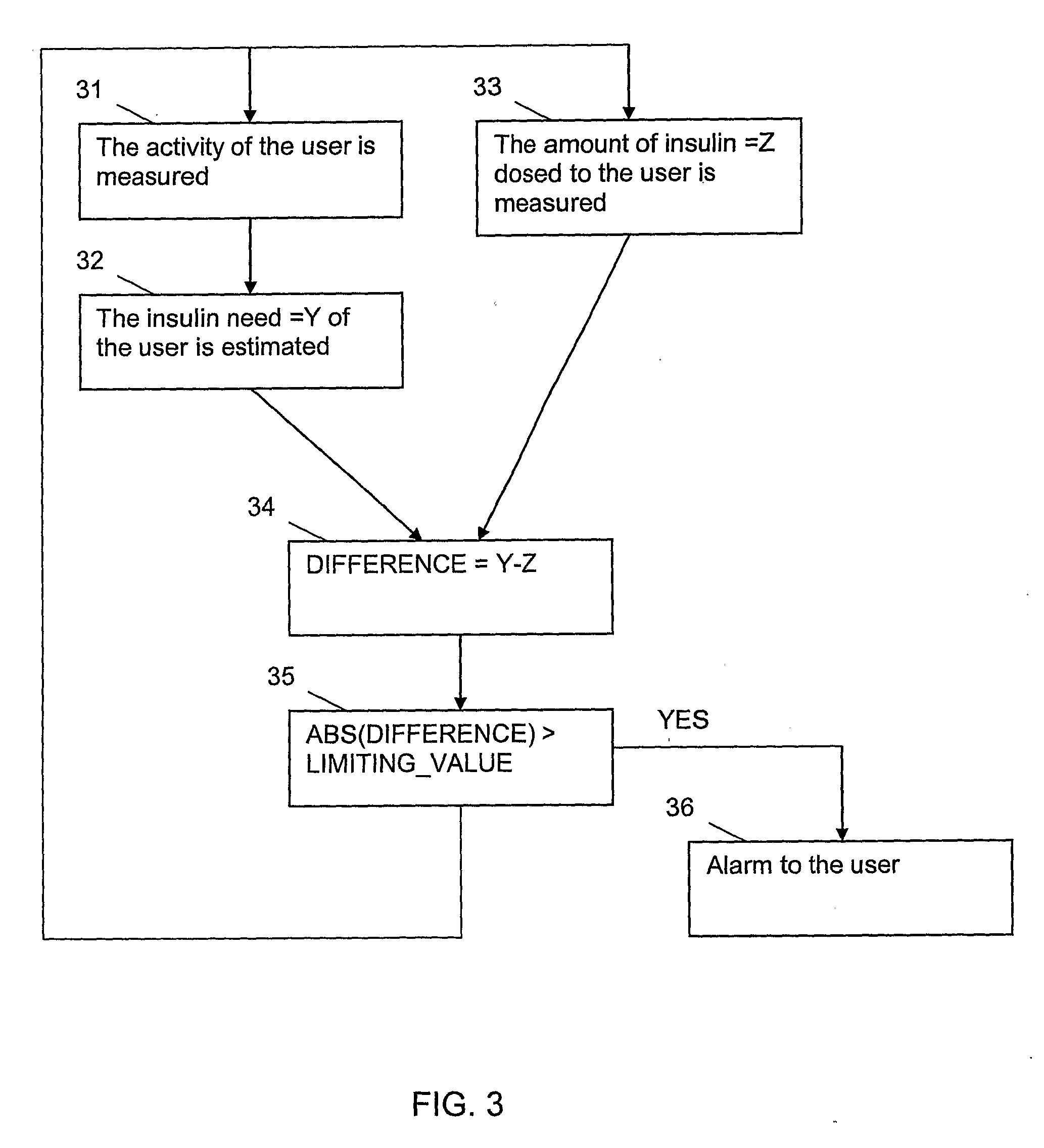
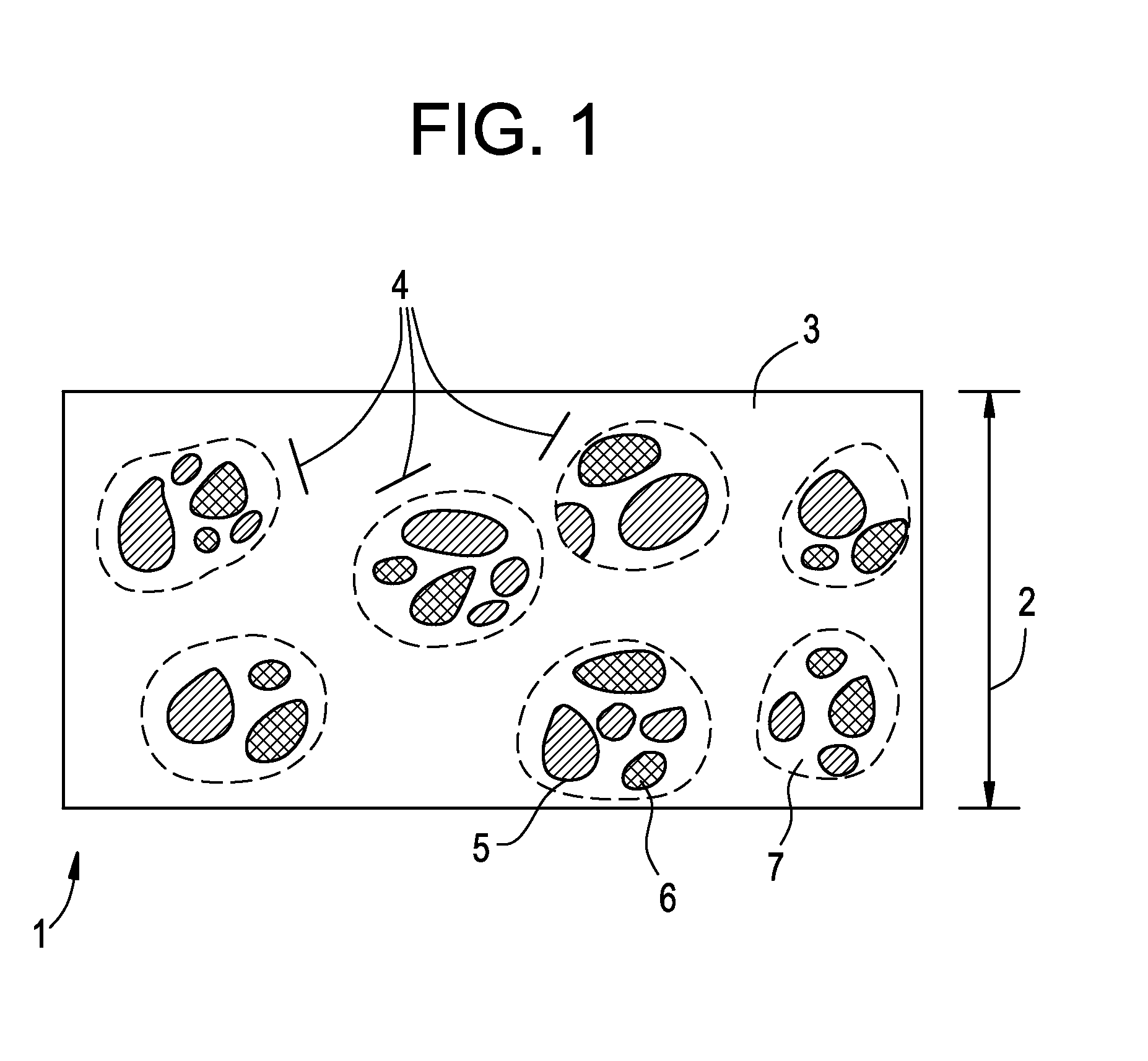
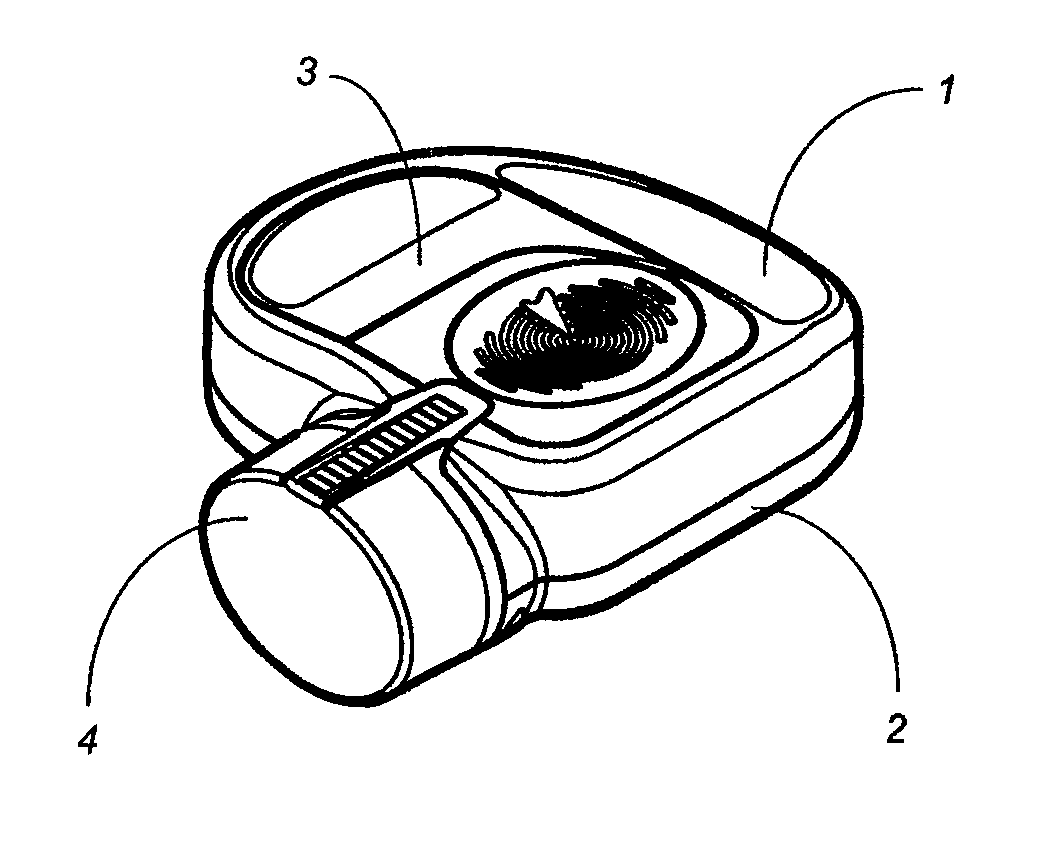
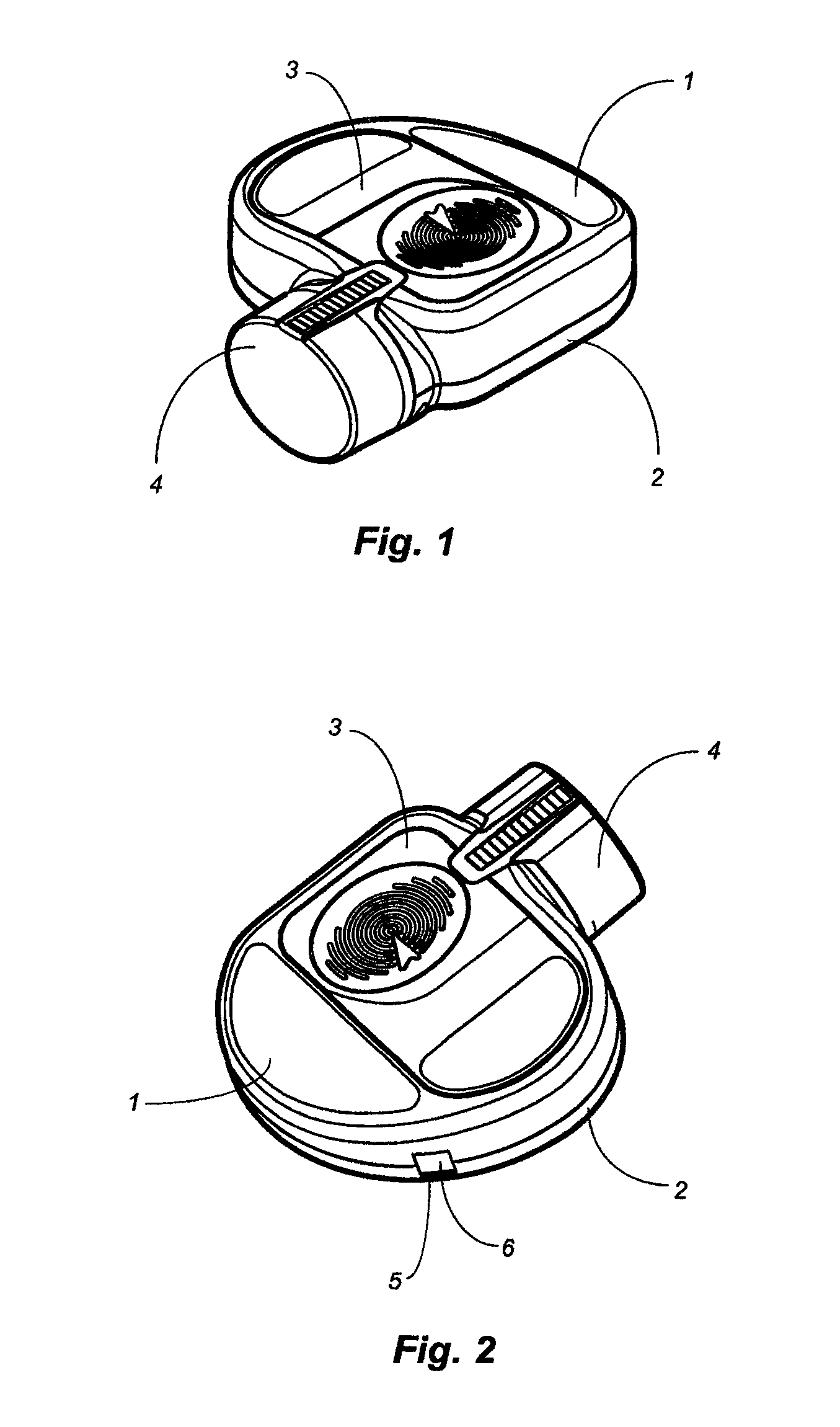
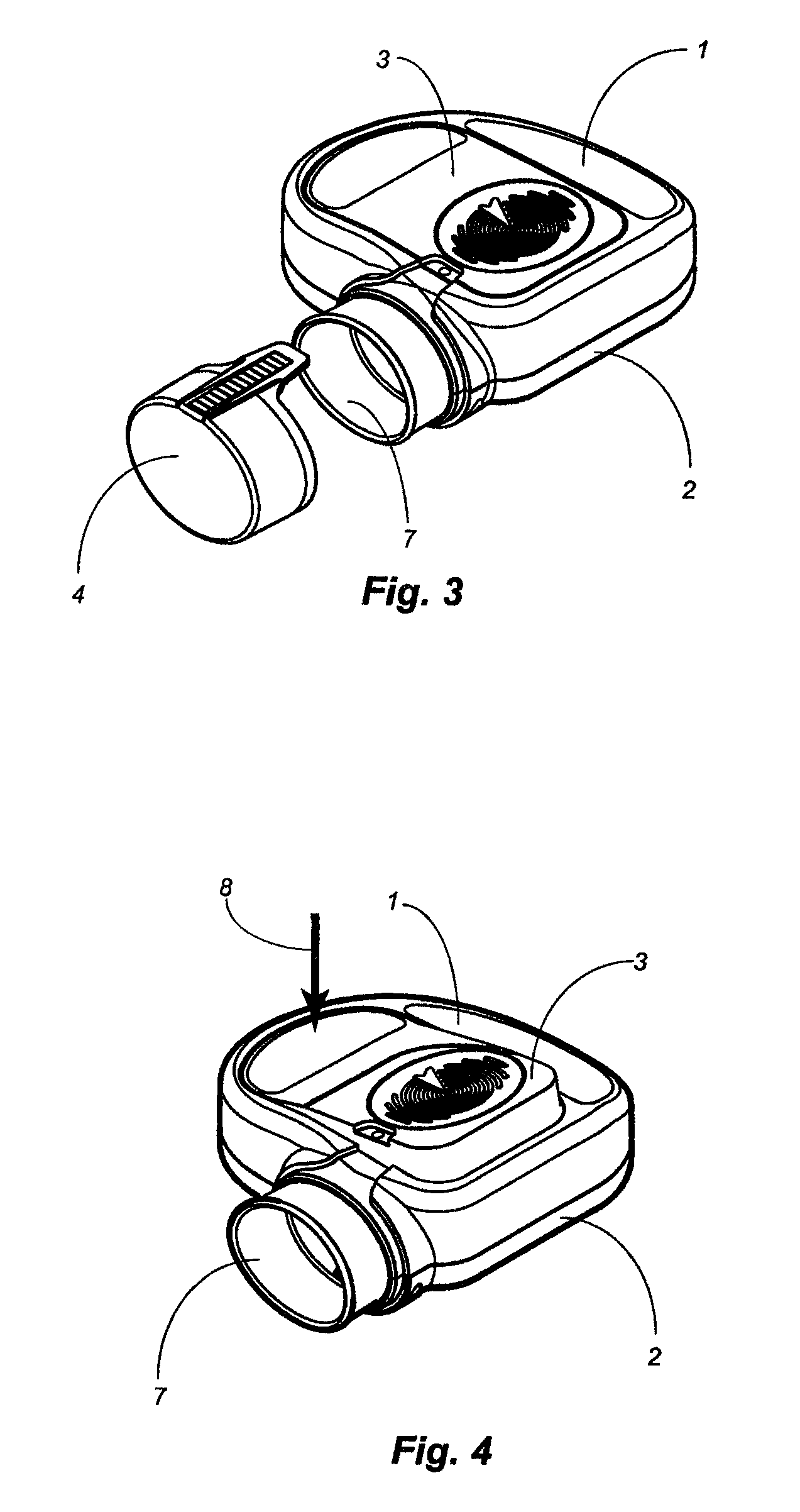
Apparatus and method for dosing drug and wireless remote control of a drug pump
InactiveUS20090209938A1Drug pumpEasy to solveElectrocardiographyDrug and medicationsMeasurement deviceRemote control
The invention comprises an apparatus meant to be carried by a user for dosing a drug, a method for dosing drug and a wireless remote control for a drug pump. The apparatus comprises a drug pump (1), a computer (9), which is arranged to control the pump, control units (10) for transmitting control functions from the user to the computer, indicators (11) for transmitting information from the computer to the user, a measuring device (12), which is arranged to produce information about the physical activity of the user, means of calculating the amount of drug needed by the user based on the information produced by the measuring device.
Owner:WRISTOP TECH
Controlled dose drug delivery system
ActiveUS20070264323A1Meet needsReduce the amount requiredOrganic active ingredientsBiocideImmediate releaseDose delivery
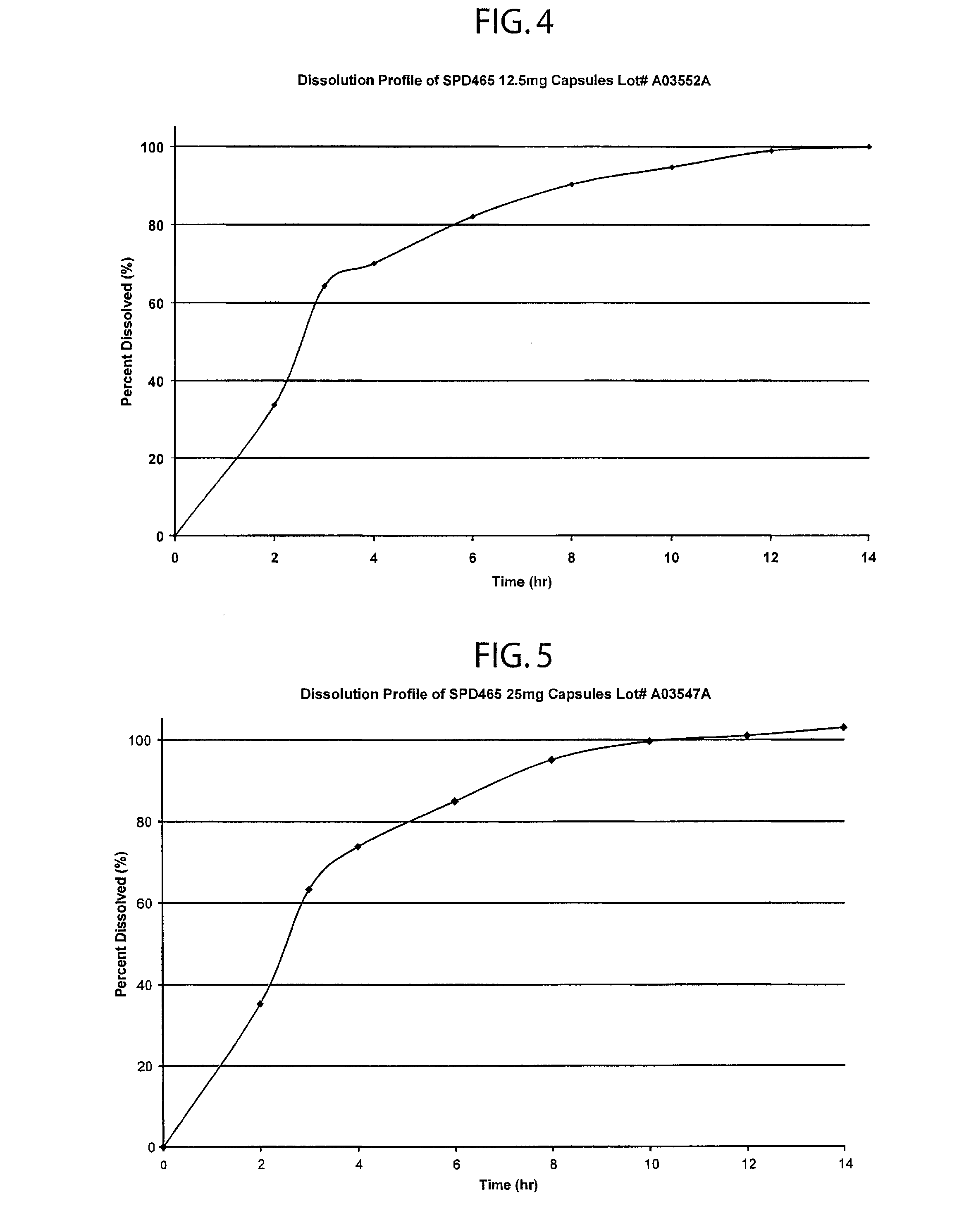
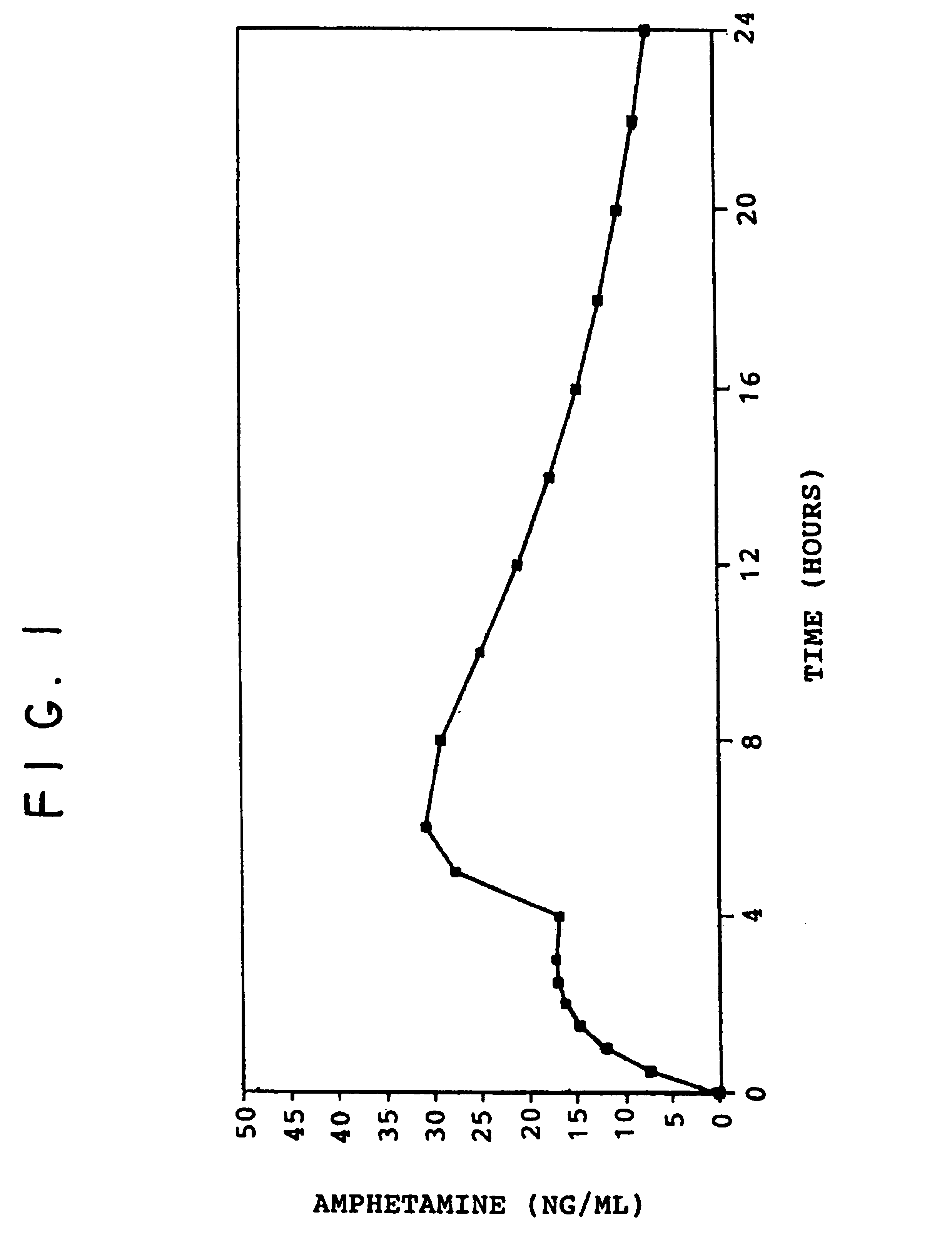
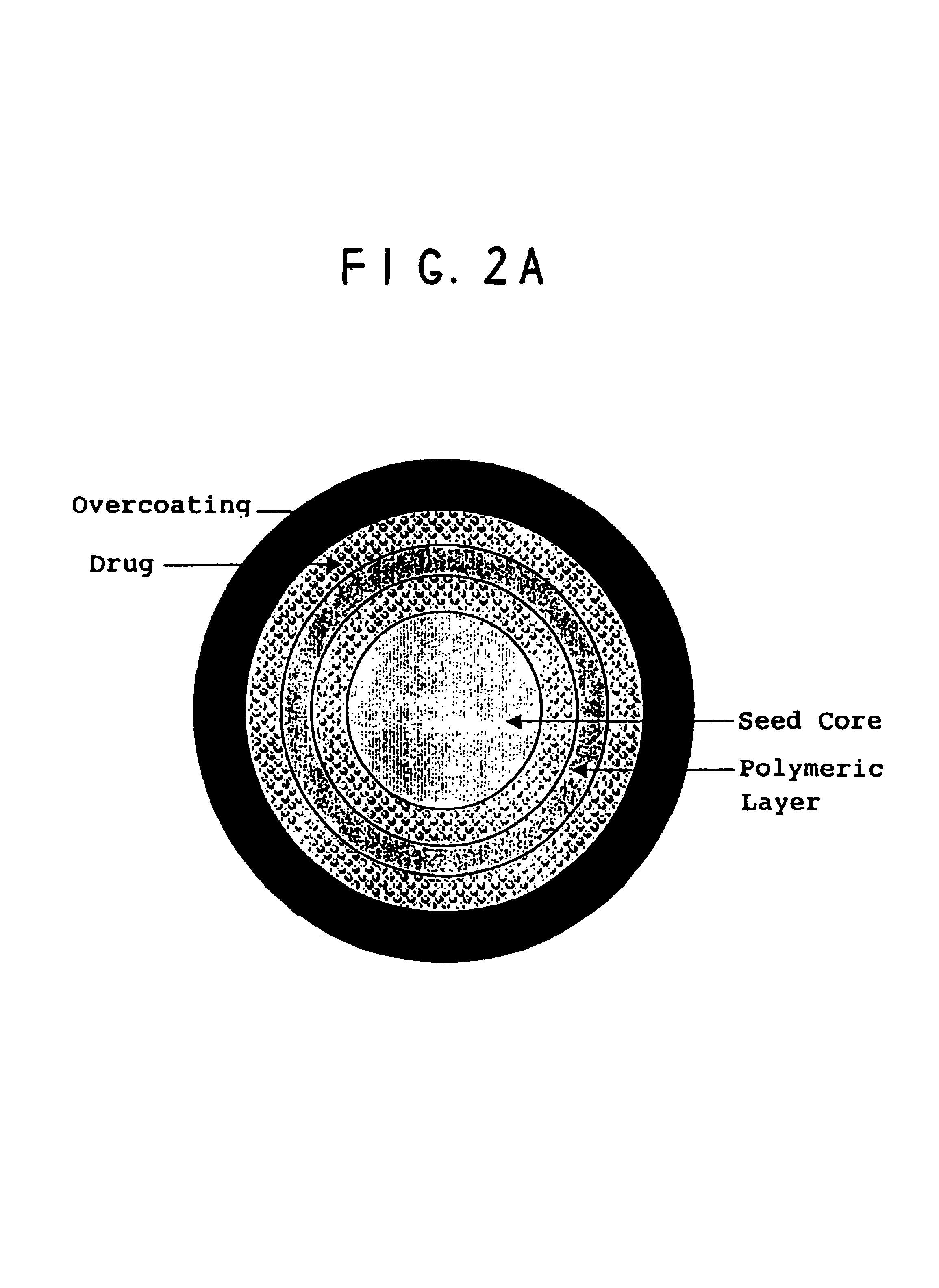
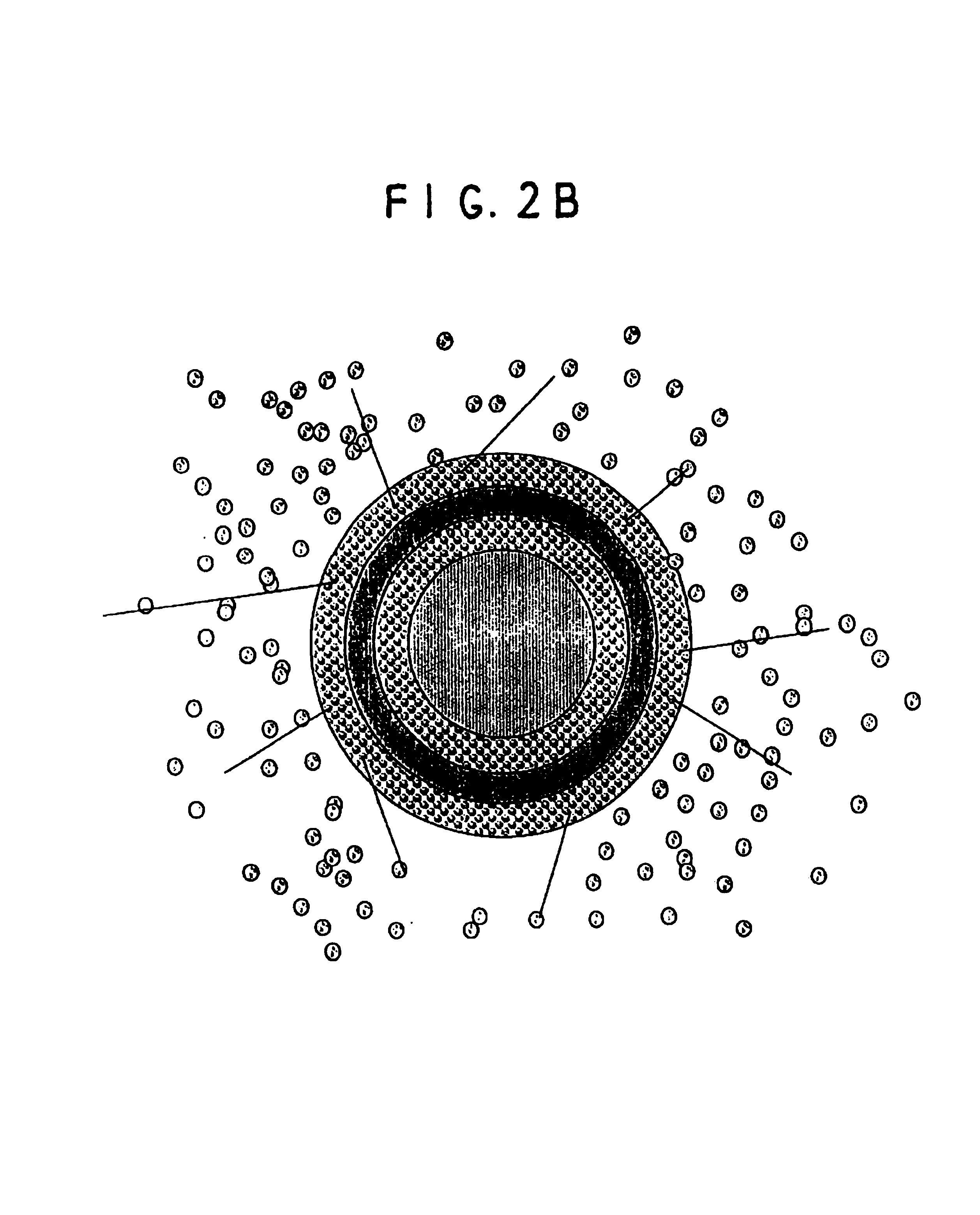
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
Multiple unit dose drug delivery system
InactiveUS20070051362A1Reduces potentially unpleasant side effectConvenient, fast and safeBiocideMedical devicesOphthalmologyNose
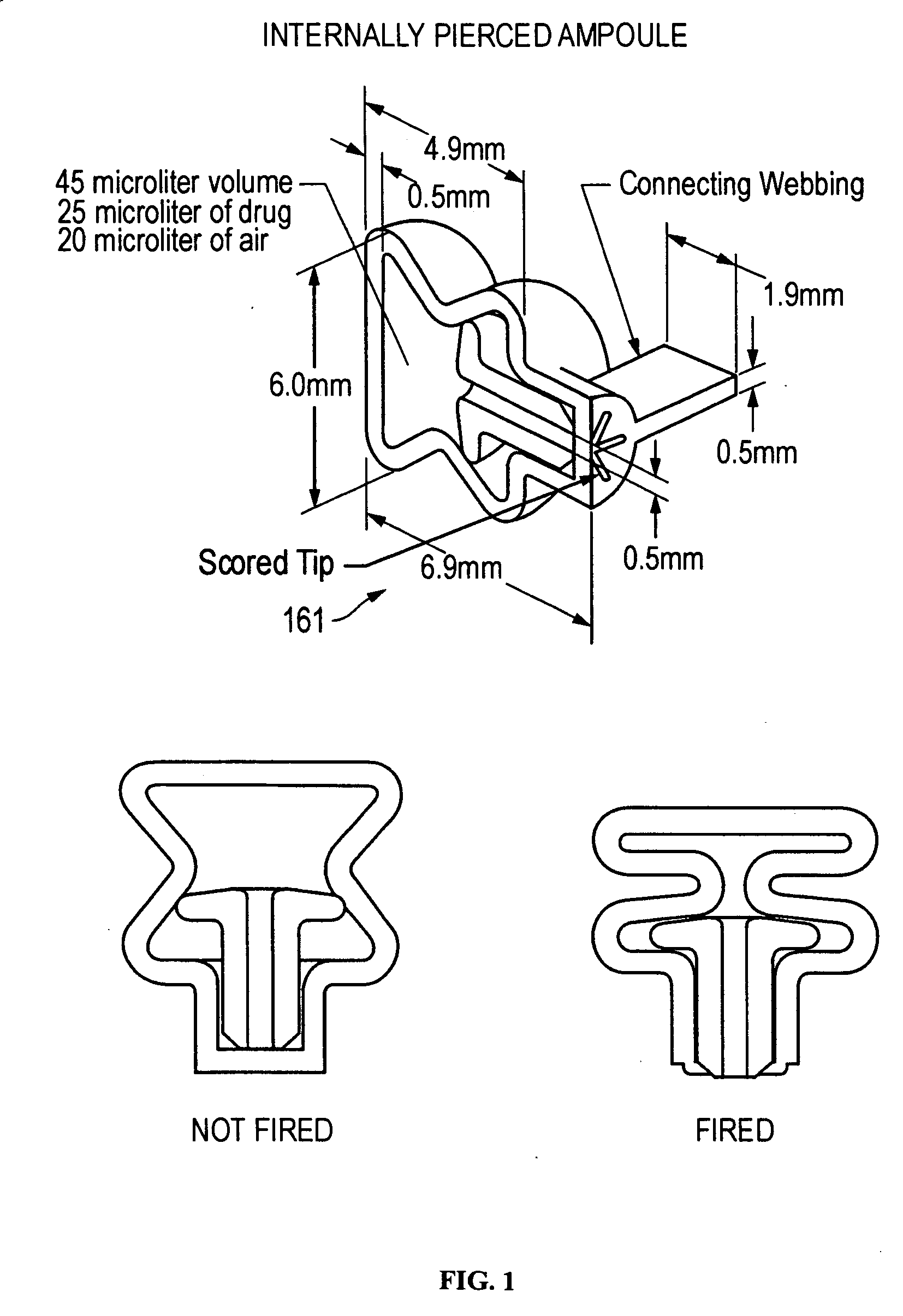
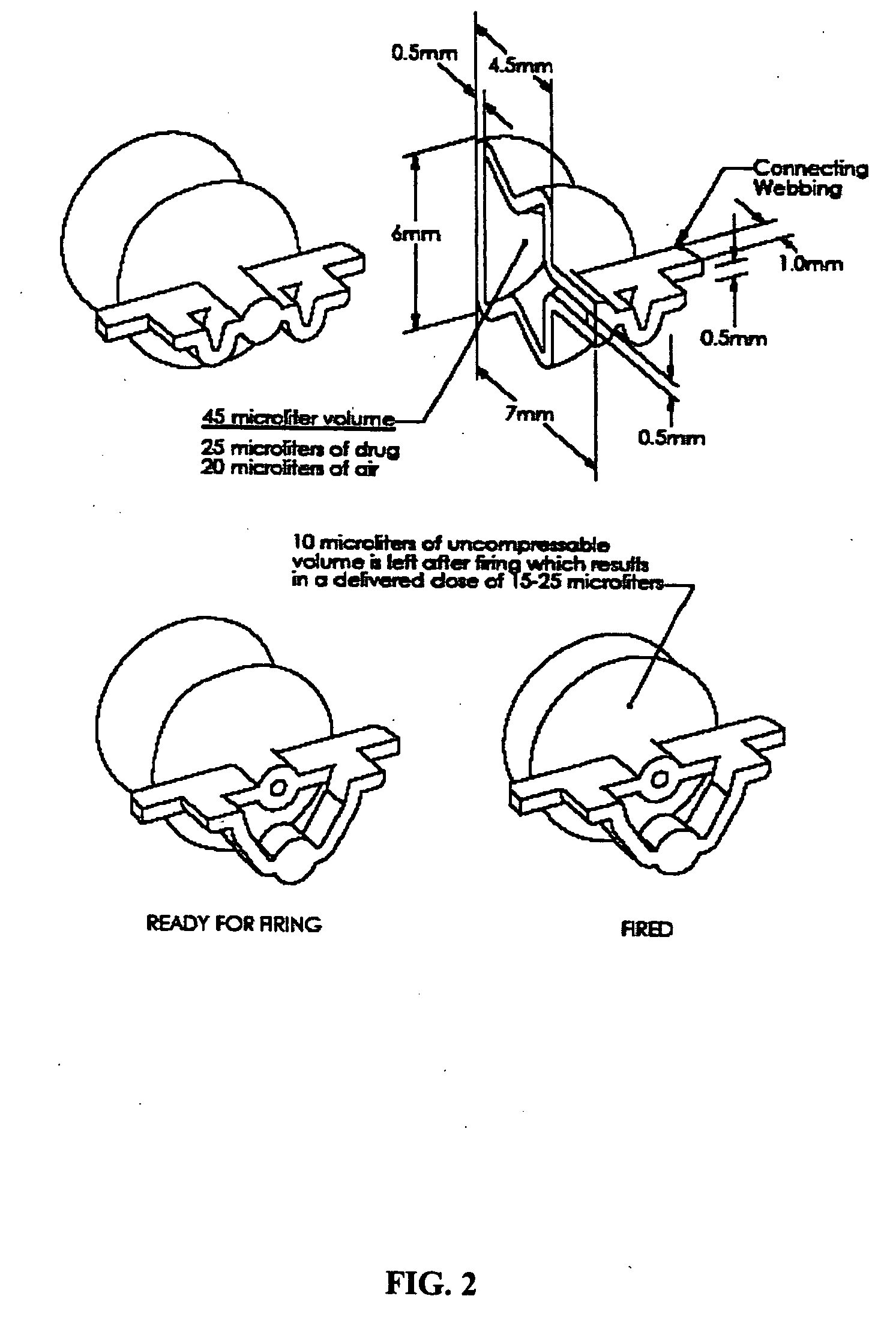
The present disclosure is directed to devices that administer single or multiple doses of one or more substances to the eye, nose, or ear of a user. The precise and repeatable dosing features of the presently disclosed devices overcome many of the disadvantages associated with known methods for dispensing substances to, for example, the eye of a user. The devices administer precise doses of a substance to a precise location from ampoules that may be single-dose or two-dose ampoules, which may be externally or internally pierced.
Owner:MYSTIC PHARMA INC
Unit Dose Drug Delivery Platform
ActiveUS20100331765A1Increase ease of administrationAdequate doseMedical devicesMedical applicatorsNoseDosing drugs
The delivery systems of the present disclosure are configurable to administer either single-dose or multiple-doses of one or more substances to a user, for example to the eye, nose, mouth, ear or rectum of the user. The precise and repeatable dosing features of the presently disclosed delivery systems overcome many of the disadvantages associated with known methods for dispensing substances to, for example, the eye of a user. The delivery systems administer precise doses of a substance to a precise location from unit dosage forms that may be single-dose or multiple-dose unit dosage forms, which may be externally or internally pierced.
Owner:MYSTIC PHARMA INC
Oral pulsed dose drug delivery system
InactiveUSRE41148E1Prolonged delayed release timeDelayed release timeBiocideOrganic active ingredientsImmediate releaseDosing drugs
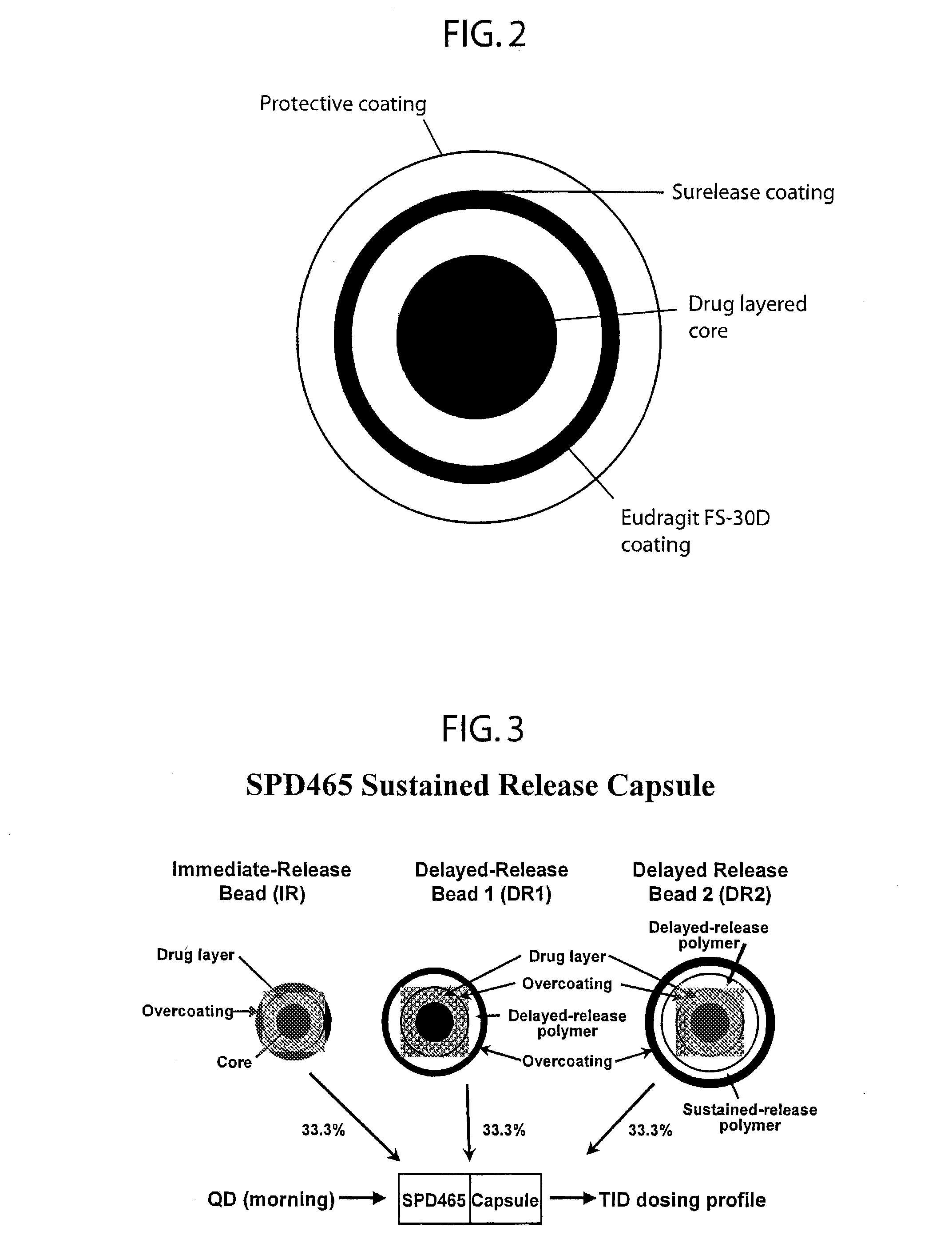
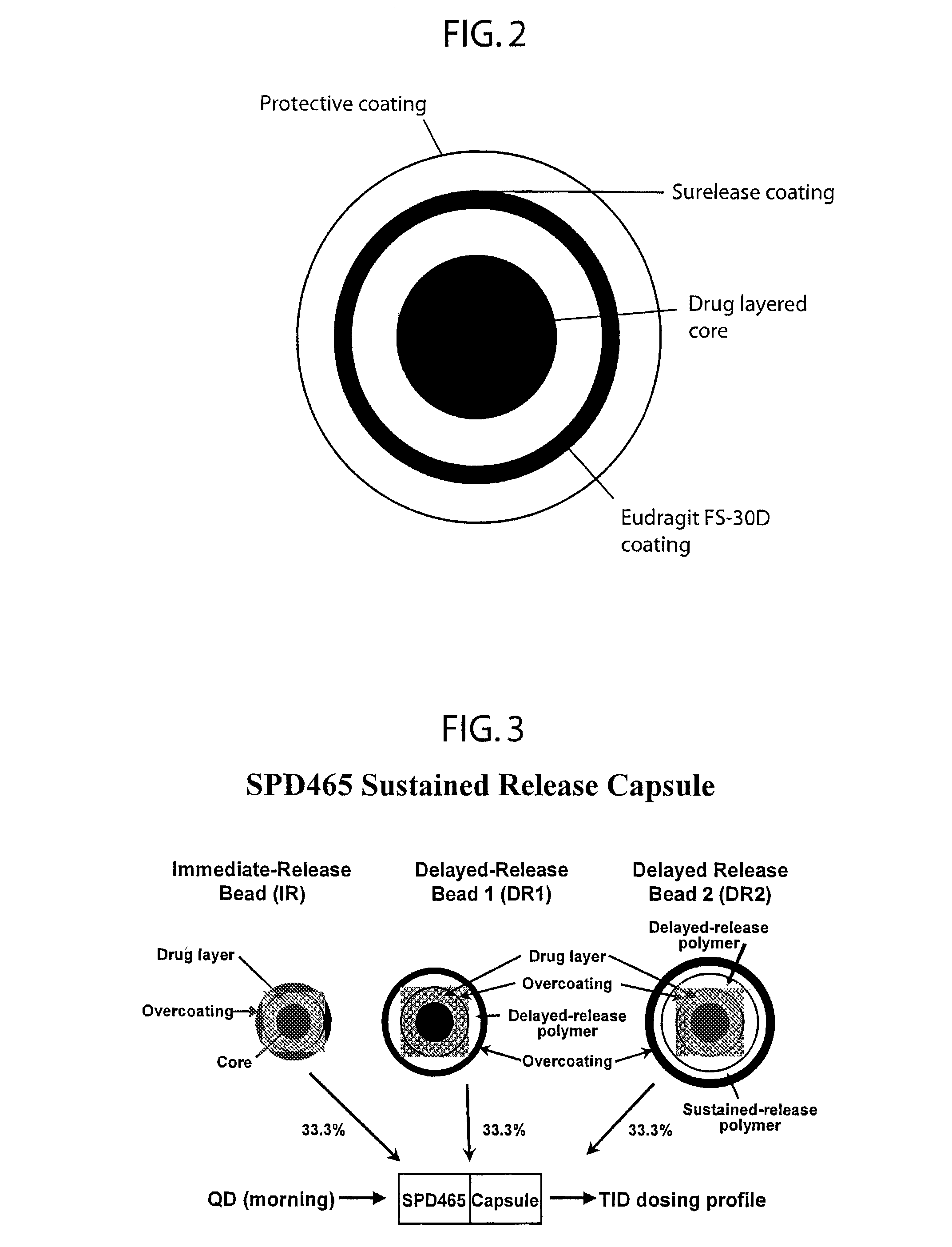
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising an immediate-release component and an enteric delayed-release component wherein (1) the enteric release coating has a defined minimum thickness and / or (2) there is a protective layer between the pharmaceutically active amphetamine salt and the enteric release coating and / or (3) there is a protective layer over the enteric release coating. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:SHIRE PLC
Controlled dose drug delivery system
ActiveUS8846100B2Meet needsReduce the amount requiredOrganic active ingredientsBiocideDose deliveryImmediate release
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising a pharmaceutically active amphetamine salt covered with an immediate-release coating and a pharmaceutically active amphetamine salt covered with an enteric coating wherein the immediate release coating and the enteric coating provide for multiple pulsed dose delivery of the pharmaceutically active amphetamine salt. The product can be composed of either one or a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:TAKEDA PHARMA CO LTD
Microneedle adaptor for dosed drug delivery devices
An adapter for achieving intradermal dosed delivery of a liquid by use of a dosed drug delivery device including a reservoir having a pierceable septum, the adapter having a connector including an attachment configuration for attachment to the dosed drug delivery device and a hollow needle deployed for piercing the septum. A liquid delivery interface, linked to the connector, includes a straight skin contact edge and one or more hollow microneedle adjacent thereto. The microneedle projects away from the skin contact edge. A flow path arrangement interconnects the needle and the at least one hollow microneedle.
Owner:NANOPASS TECH LTD
Oral pulsed dose drug delivery system
InactiveUSRE42096E1Control erosionDelayed release timePowder deliveryOrganic active ingredientsCaplet Dosage FormImmediate release
A multiple pulsed dose drug delivery system for pharmaceutically active amphetamine salts, comprising an immediate-release component and an enteric delayed-release component wherein (1) the enteric release coating has a defined minimum thickness and / or (2) there is a protective layer between the pharmaceutically active amphetamine salt and the enteric release coating and / or (3) there is a protective layer over the enteric release coating. The product can be composed of either one of a number of beads in a dosage form, including either capsule, tablet, or sachet method for administering the beads.
Owner:SHIRE PLC
Oral Film Containing Opiate Enteric-Release Beads
A control release and abuse-resistant opiate drug delivery oral wafer or edible oral film dosage to treat pain and substance abuse is provided. The drug delivery oral wafer or edible oral film dosage includes a controlled release layer containing enteric-release beads dispersed in a polymer matrix. The enteric-release beads are formed from a therapeutic amount of an opioid agonist and / or pharmaceutically acceptable salt thereof and a sub-therapuetic amount of opioid antagonist and / or pharmaceutically acceptable salt thereof coated or encapsulated in an enteric-release polymer. The controlled release polymer matrix dissolves or disintegrates following administration or consumption of the oral wafer or edible oral film, releasing the enteric-release beads to be swallowed, with subsequent absorption of the active ingredients within the patient's intestines.
Owner:LTS LOHMANN THERAPIE-SYST AG
Method for customized dispensing of variable dose drug combination products for individualizing of therapies
The method set out herein involves identifying the concentration of each of two or more active therapeutics tailored to treat a particular patient's unique metabolism and one or more diseases, communicating that information to a producer who has multiple fixed or variable concentrations of each active available, where the producer then combines the individual concentrations of each active into single units such as a tablets or pills, and distributes those indirectly or directly to the patient.
Owner:GLAXO SMITHKLINE LLC
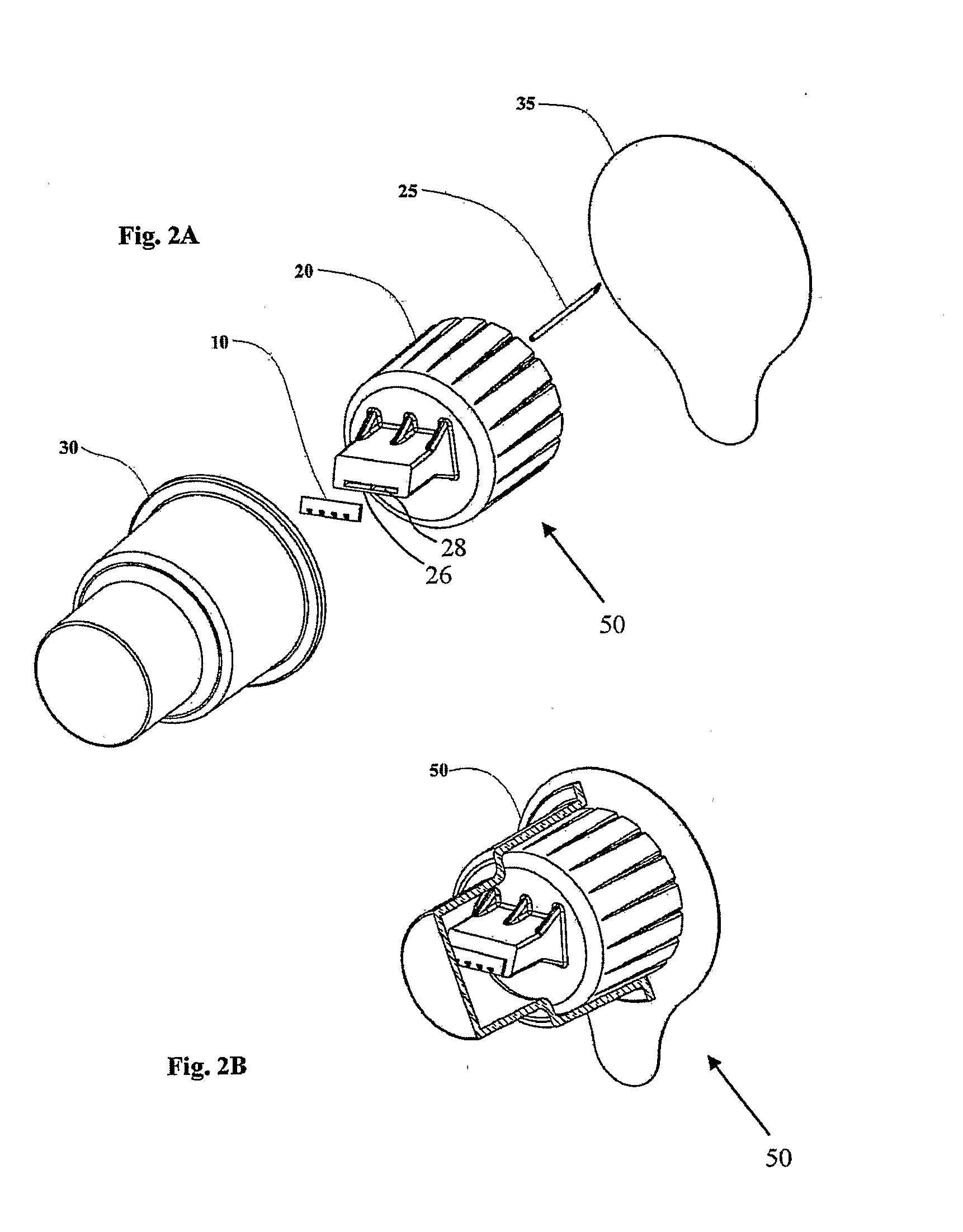
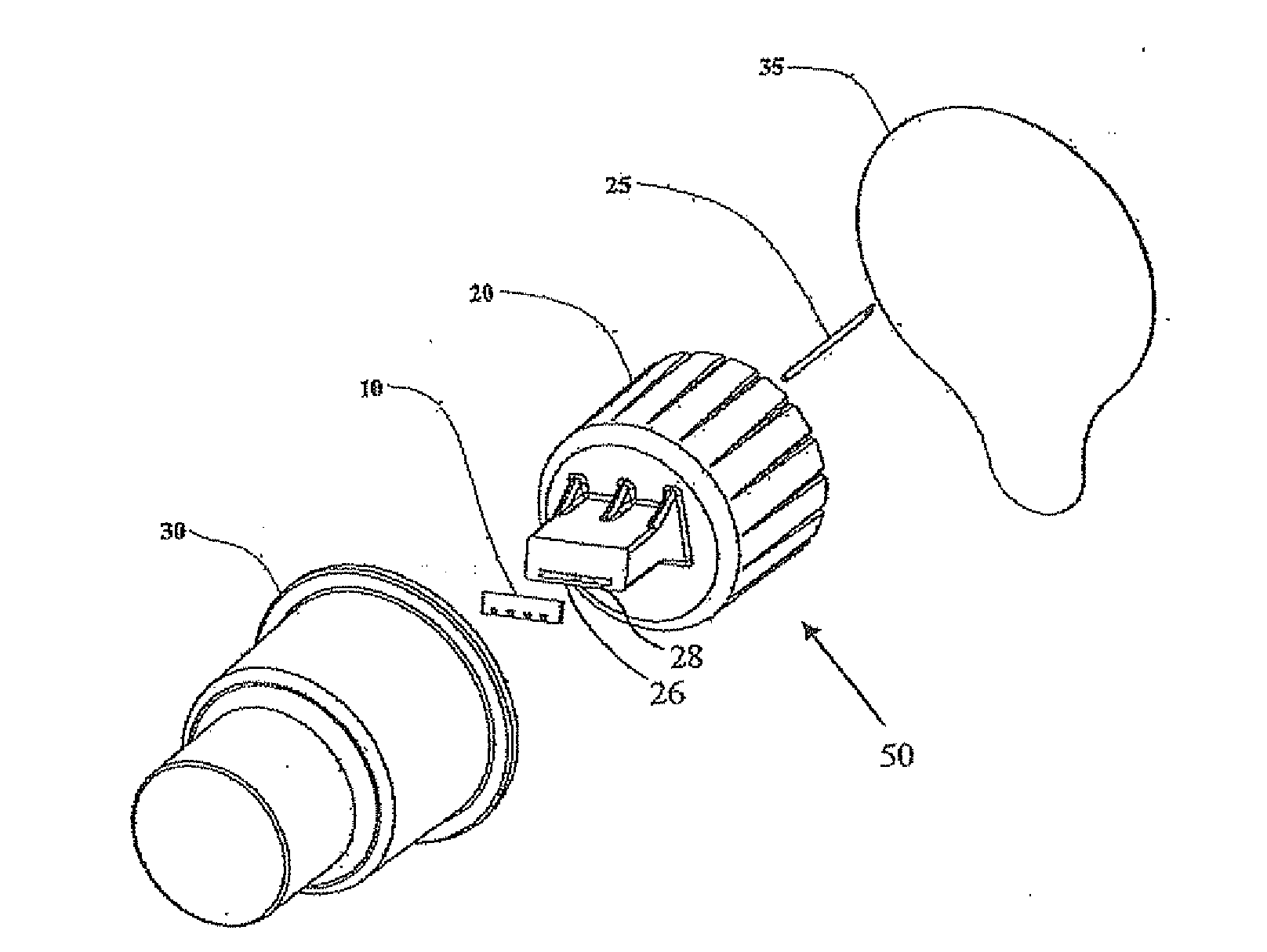
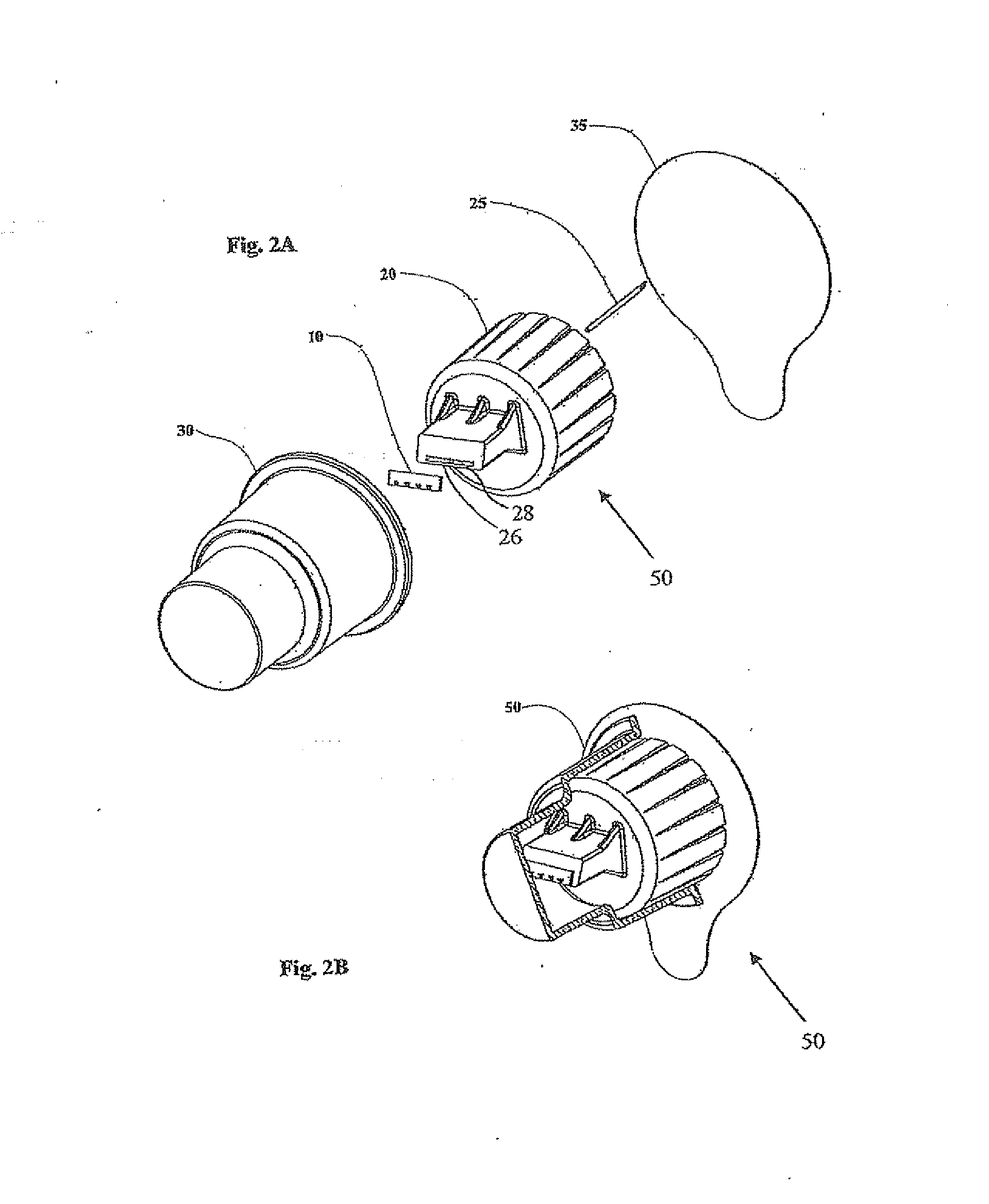
Microneedle adapter for dosed drug delivery devices
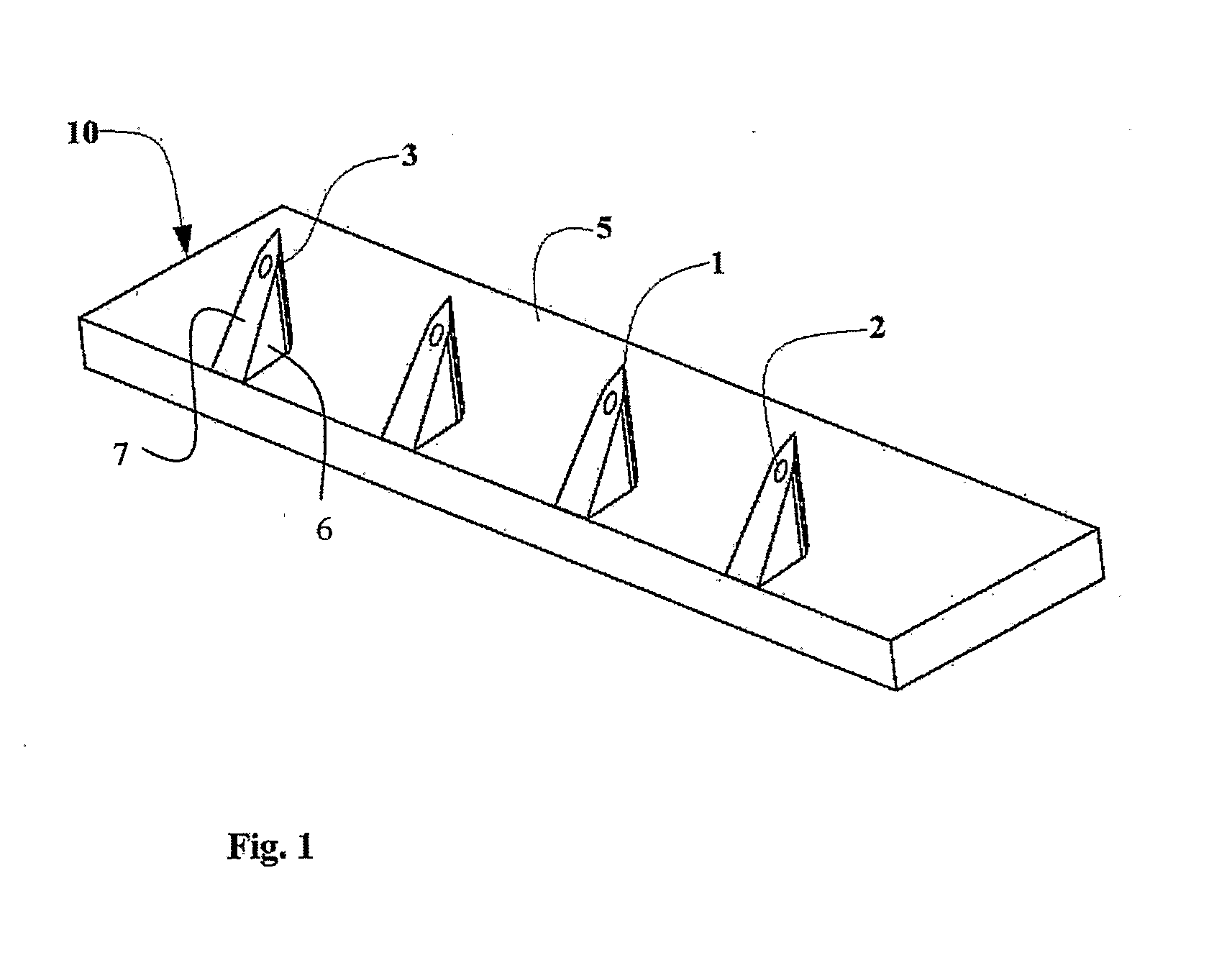
An adapter (50) for achieving intradennal dosed delivery of a liquid by use of a dosed drug delivery device including a reservoir having a pierceable septum, the adapter having a connector including an attachment configuration (22) for sliding a recess of the adapter onto a projection of the dosed drug delivery device and a hollow needle (25) deployed for piercing the septum. At least one microneedle (10) projects from the connector. A flow path arrangement (28) interconnects the needle and the hollow microneedle(s). The recess is shaped to restrict the microneedle(s) to only a finite number of rotational orientations relative to the axis of the device. The connector and / or the needle is adapted for application of a sealant to prevent leakage of the liquid.
Owner:NANOPASS TECH LTD
Combination containing chitosan oligosaccharide and ipconazole or prothioconazole
InactiveCN104782633AImprove the effect of disease preventionSynergistic effect is obviousPlant growth regulatorsBiocideDiseaseMedicine
The invention belongs to the field of plant protection, and relates to an antibacterial combination containing chitosan oligosaccharide. The combination is characterized by comprising a constituent A and a constituent B, wherein the constituent A adopts chitosan oligosaccharide; the constituent B adopts ipconazole or prothioconazole. The combination shows good synergistic effect within certain matching range, is higher in the disease prevention effect, can prevent and treat various diseases in agricultural production, is lower in use frequency and use cost, and delays the occurrence of single-dose drug resistance of pathogen.
Owner:HAINAN ZHENGYE ZHONGNONG HIGH TECH
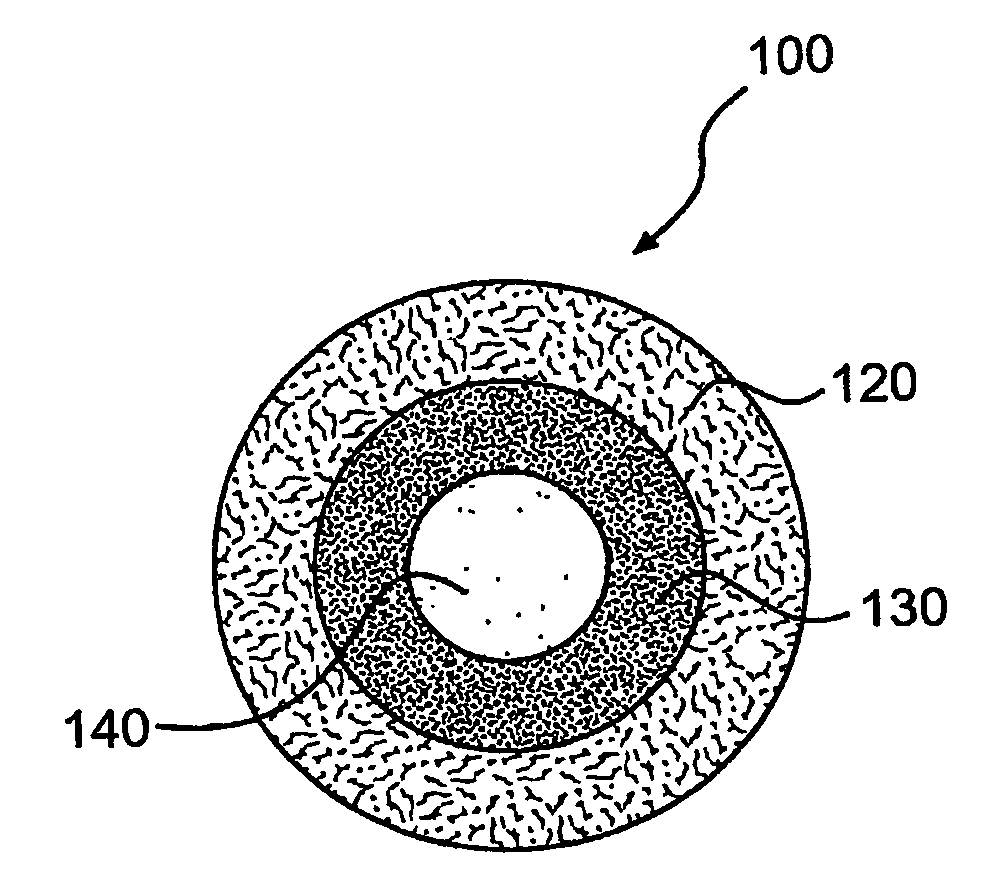
Formulations of human tissue kallikrein-1 for parenteral delivery and related methods
InactiveUS20130315891A1Improved systemic pharmacokineticsImprove bioavailabilityPeptide/protein ingredientsMetabolism disorderKininDosing drugs
Provided are high concentration compositions of tissue kallikrein-1 (KLK1) and methods of parenterally administering such compositions to a subject in need thereof, where absorption into the circulation via, for example, intravenous or subcutaneous administration improves systemic pharmacokinetics, bioavailability, safety, and / or convenience relative to intravenous or other forms of administration. Also provided are recombinant human KLK1 (rhKLK1) polypeptides that can be readily concentrated to high protein concentrations, and substantially pure compositions thereof.
Owner:DIAMEDICA INC
Unit dose drug delivery platform
ActiveUS8579856B2Increase ease of administrationOvercome problemsMedical devicesMedical applicatorsMedicineNose
Owner:MYSTIC PHARMA INC
Drug dispensing terminal capable of prompting drug cup position and prompting method of drug dispensing terminal
PendingCN108320784AImprove work efficiencyReduce workloadDrug and medicationsRigid containersDrug dispensingMedicine
The invention discloses a drug dispensing terminal capable of prompting a drug cup position and a prompting method of the drug dispensing terminal. The drug dispensing terminal comprises a tray, wherein a plurality of drug cups for storing patient single dose drugs are arranged on the tray; the tray is provided with a position prompting unit for prompting the drug cup position of some patient single dose drug; the position prompting unit comprises at least one of a lamplight prompting device, a sound prompting device and a lifting prompting device; and the terminal improves the drug dispensingwork efficiency of medical personnel and reduces the workload of the medical personnel.
Owner:TIANJIN SENYA MEDICAL EQUIP TECH CO LTD
Oral film containing opiate enteric-release beads
A control release and abuse-resistant opiate drug delivery oral wafer or edible oral film dosage to treat pain and substance abuse is provided. The drug delivery oral wafer or edible oral film dosage includes a controlled release layer containing enteric-release beads dispersed in a polymer matrix. The enteric-release beads are formed from a therapeutic amount of an opioid agonist and / or pharmaceutically acceptable salt thereof and a sub-therapeutic amount of opioid antagonist and / or pharmaceutically acceptable salt thereof coated or encapsulated in an enteric-release polymer. The controlled release polymer matrix dissolves or disintegrates following administration or consumption of the oral wafer or edible oral film, releasing the enteric-release beads to be swallowed, with subsequent absorption of the active ingredients within the patient's intestines.
Owner:LTS LOHMANN THERAPIE-SYST AG
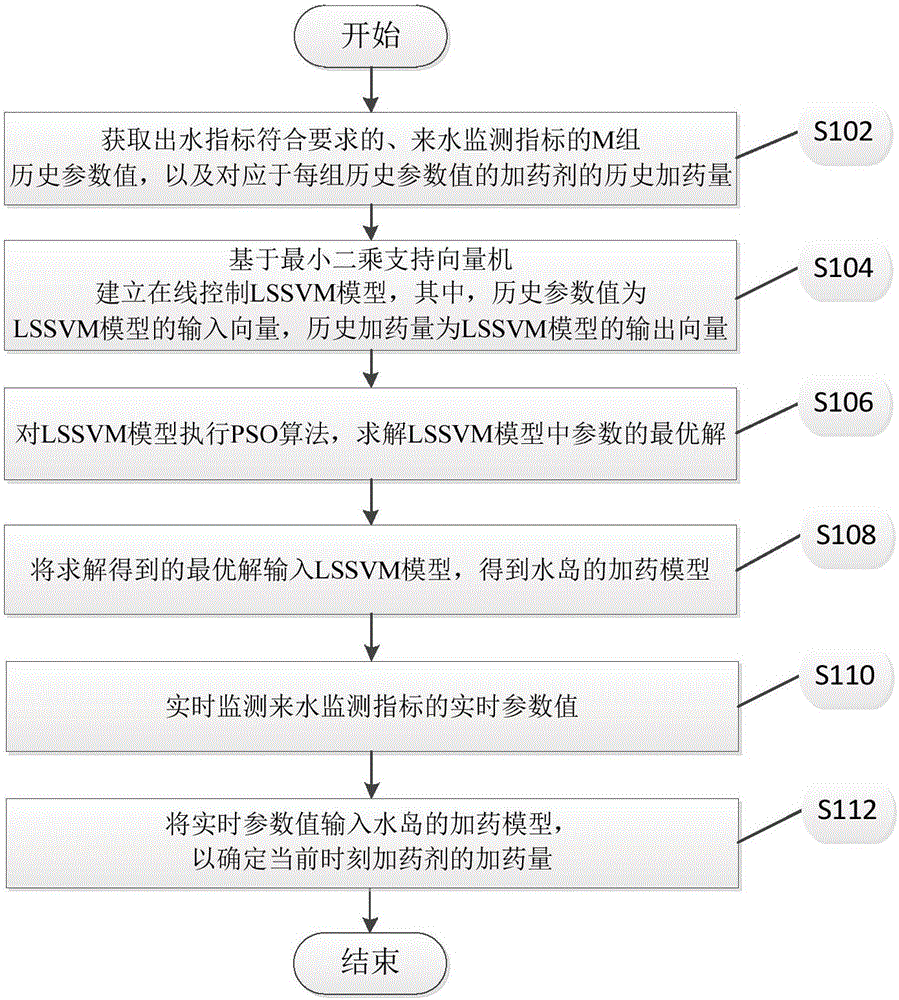
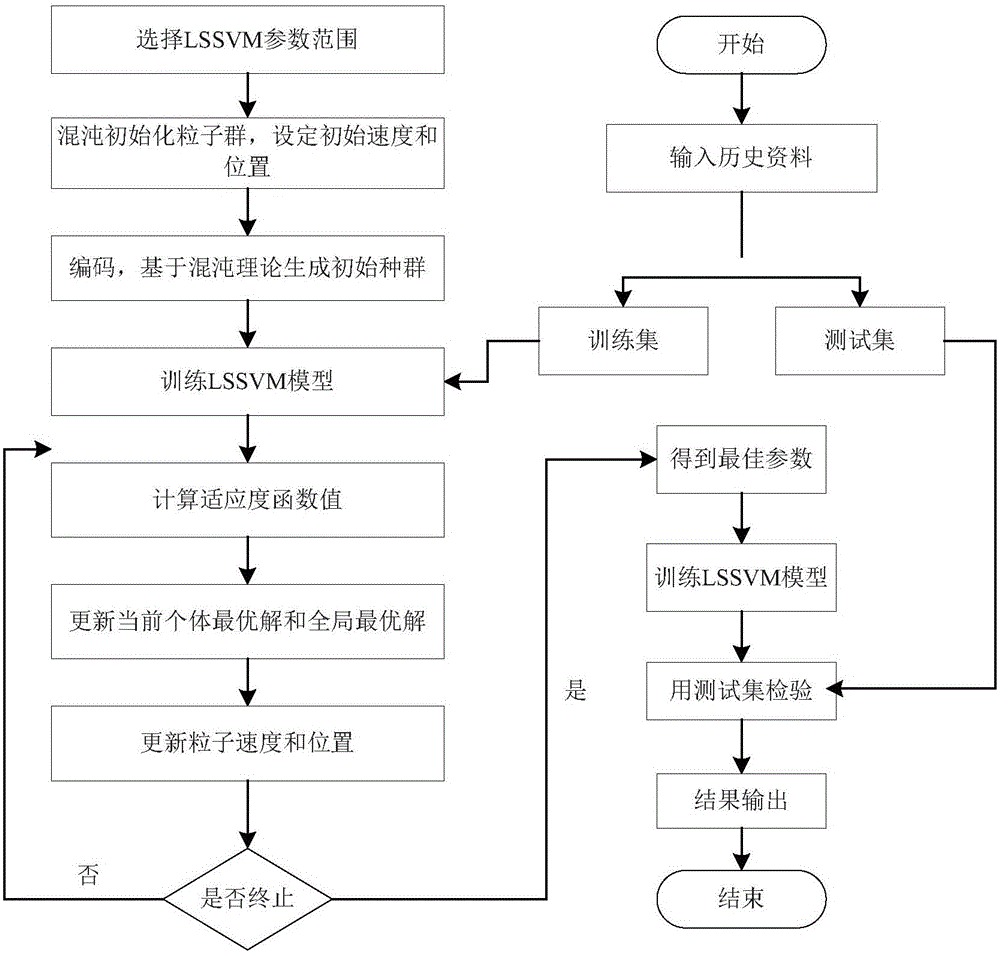
PSO-LSSVM-based on-line control method and apparatus for dosing of water island
ActiveCN106371316AReduce wasteRealize online controlAdaptive controlWater qualityLeast squares support vector machine
The invention discloses a PSO-LSSVM-based on-line control method and apparatus for dosing of a water island. The method comprises: M groups of historical parameter values with outgoing water indexes meeting requirements of an incoming water monitoring index as well as a historical dosing amount of a dosing drug corresponding to each historical parameter value group are obtained; on the basis of a least-squares support vector machine, an on-line control LSSVM model is established, wherein the historical parameter values are input vectors of the LSSVM model and historical dosing amounts are output vectors of the LSSVM model; a PSO algorithm is executed on the LSSVM model and an optimal solution of parameters in the LSSVM model is calculated; and the calculated optimal solution is inputted into the LSSVM model to obtain a dosing model of a water island; an incoming water monitoring index is monitored in real time to obtain a group of real-time parameter values; and real-time parameter values are inputted into the dosing model of the water island and the dosing amounts of the dosing drugs at a current time are determined. With the method, the influence on the operating process by the quality of incoming water can be eliminated; the dosing amount is updated in real time; the drug wasting is reduced; and thus costs are lowered.
Owner:大唐(北京)水务工程技术有限公司
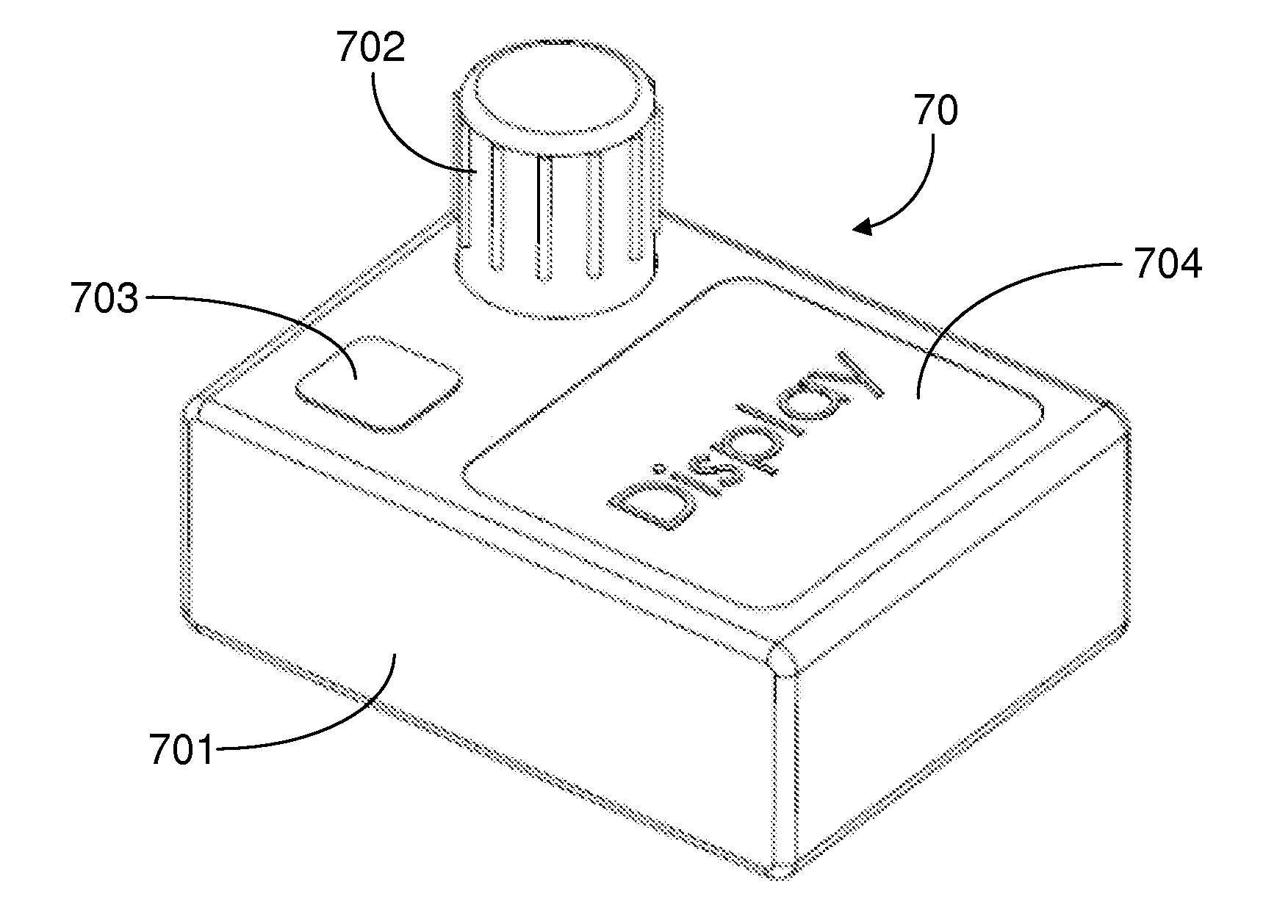
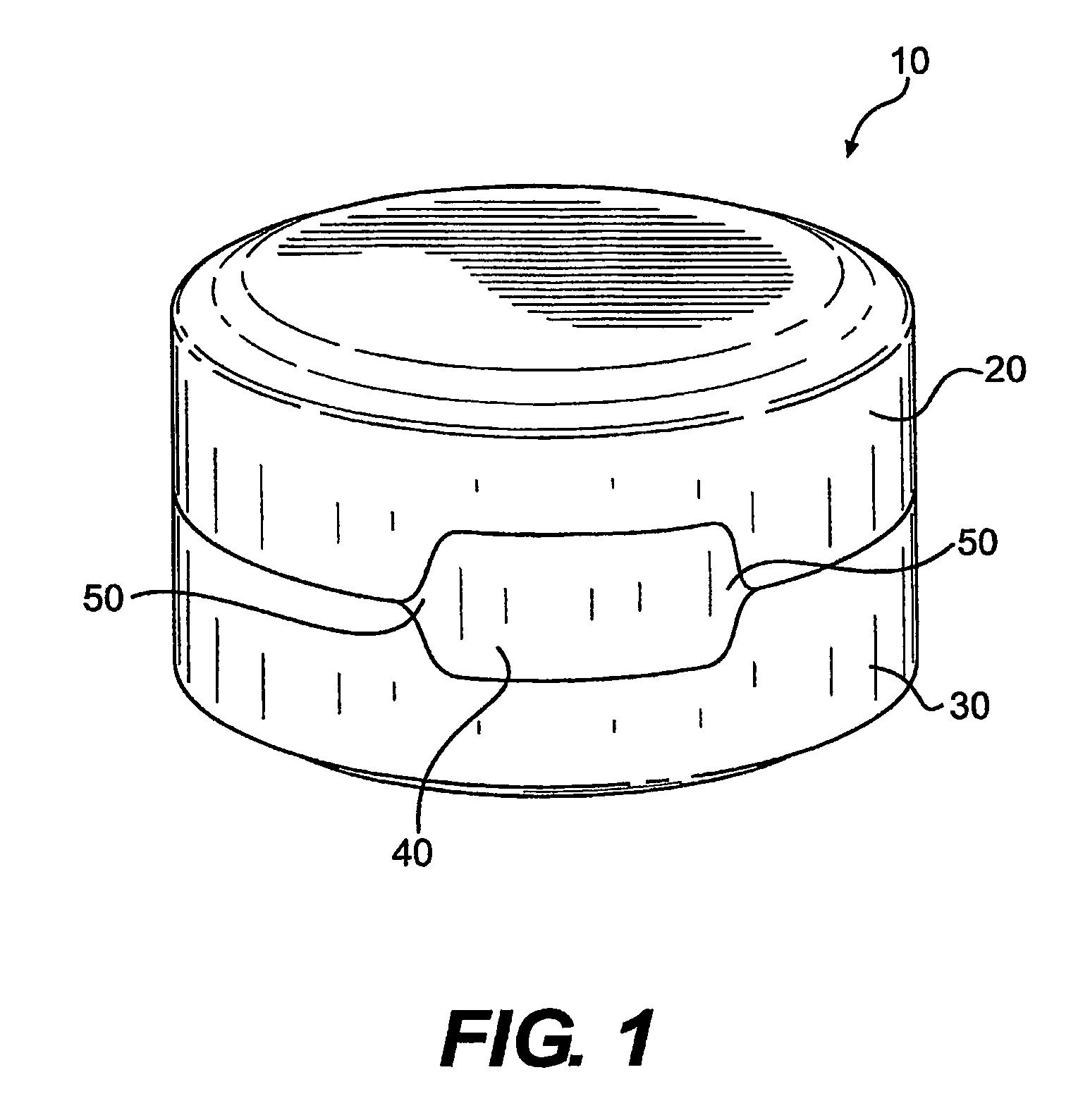
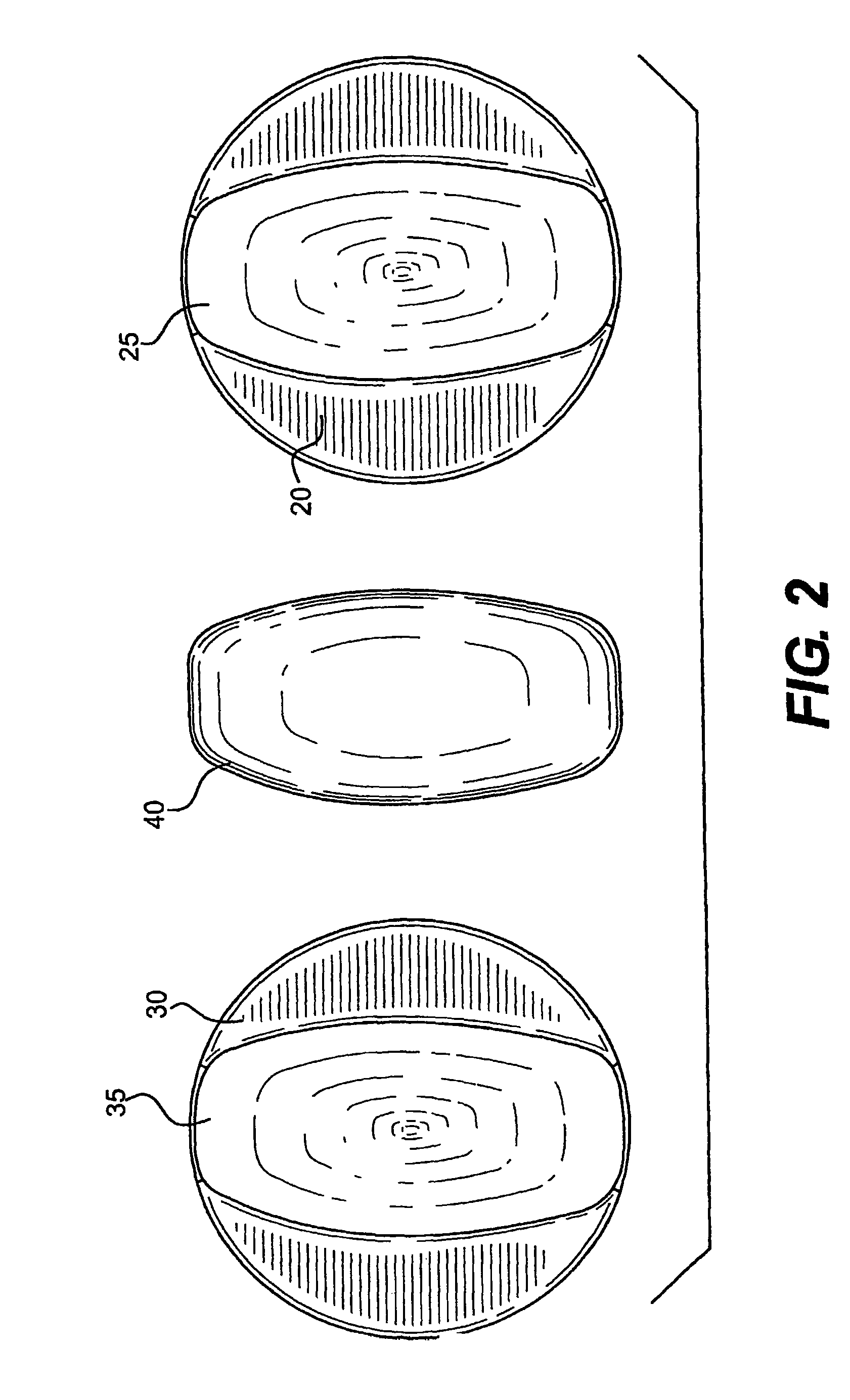
Drug and food storage and distribution bottle
InactiveCN101700208AImprove work efficiencyReduce labor intensitySmall article dispensingRacksFood safetyDosing drugs
The invention provides a drug and food storage and distribution bottle comprising a cover and a body, wherein the cover is provided with a button; a drug dropping mechanism is arranged in a tube-like cavity which is arranged at the lower part of the button and is connected with the body; the lower part of the body is provided with a drug dropping opening; the cover is arranged on the upper part of the body to form a whole; and the drug dropping mechanism is driven to drop single-dose drugs from the drug dropping opening by pressing the button on the cover. The drug and food storage and distribution bottle of the invention is provided with the unique single-dose drug dropping mechanism, so that users can take out the drugs or food in the bottle by single dose only by pressing the button on the cover. The bottle is simple and convenient in operation and can ensure the drugs or food which is put in first is taken out, thus ensuring the safety of the drugs and food, simultaneously increasing the working efficiency and reducing the labor intensity.
Owner:BEIJING OUMAI CENTURY SCI & TECH
Composition of excipients and pharmaceutical forms with sustained release and increased bioavailability of antibacterial drugs, anticoccidial drugs and other drugs for commercial poultry and pigs
InactiveUS20160279063A1Reduce wasteImprove durabilityAntibacterial agentsTetracycline active ingredientsActive agentPharmacodynamic Study
The invention relates to a composition of excipients and pharmaceutical forms of sustained release and increased drug bioavailability for poultry and pigs and to a method of producing the same, said composition comprises: pharmaceutically active agents, bioavailability promoting agents, polymers for prolonged release of the drug, colouring agents, and flavouring agents. The composition of excipients and pharmaceutical forms of the invention optimizes the dosing of the drug dosage and generates resistant strains of bacteria by optimizing the ratio between pharmacokinetics / pharmacodynamics of drugs. The composition has different forms and colours that allow the product to be identified and accepted more easily by the bird or pig.
Owner:UNIV NAT AUTONOMA DE MEXICO
Method for customized dispensing of variable dose drug combination products for individualizing of therapies
Generally the method involves identifying the concentration of each of two or more active therapeutics tailored to treat a particular patient's unique metabolism and one or more diseases, communicating that information to a producer who has multiple fixed or variable concentrations of each active available, where the producer then combines the individual concentrations of each active into single units such as a tablets or pills, and distributes those indirectly or directly to the patient.
Owner:GLAXO SMITHKLINE LLC
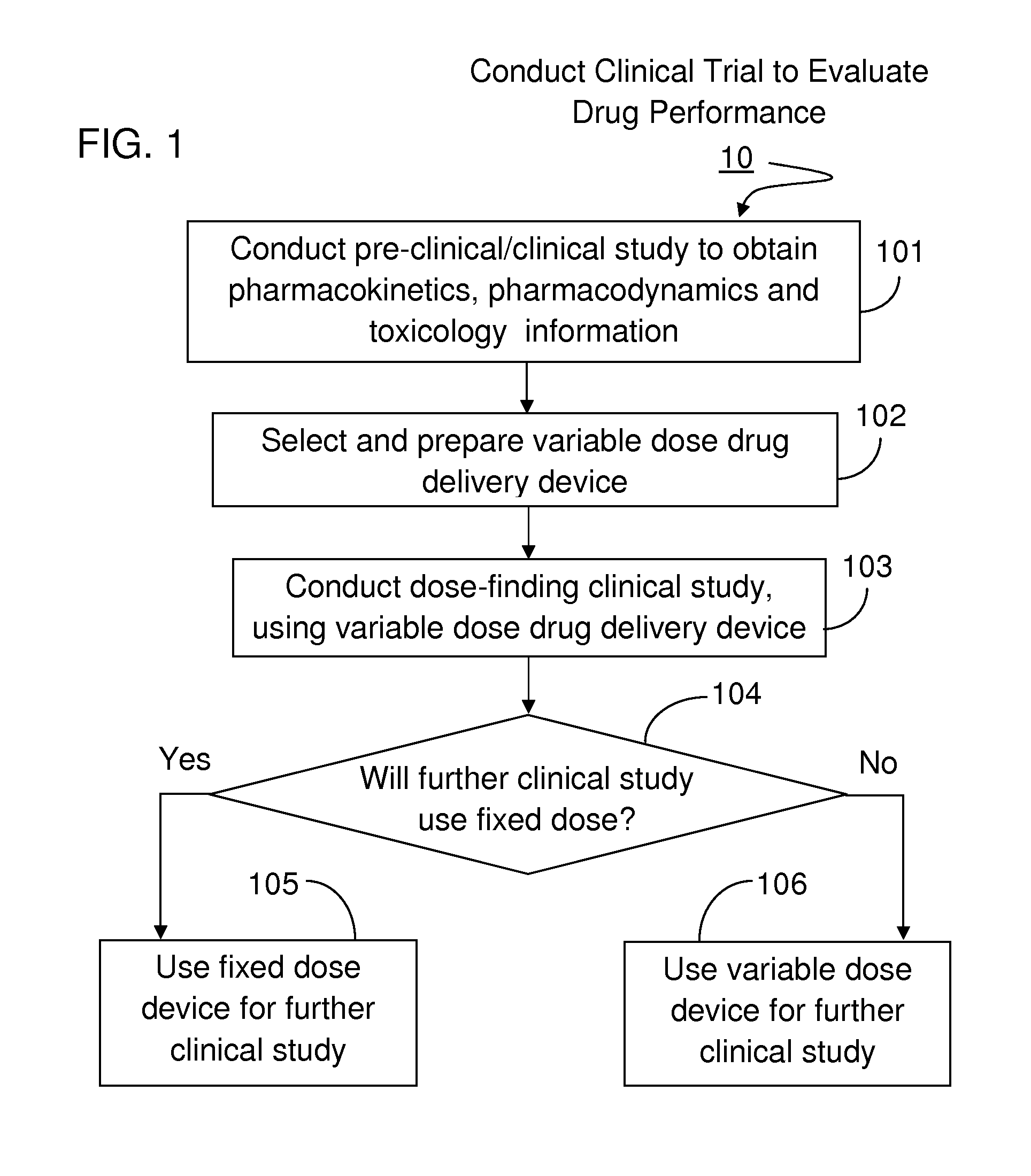
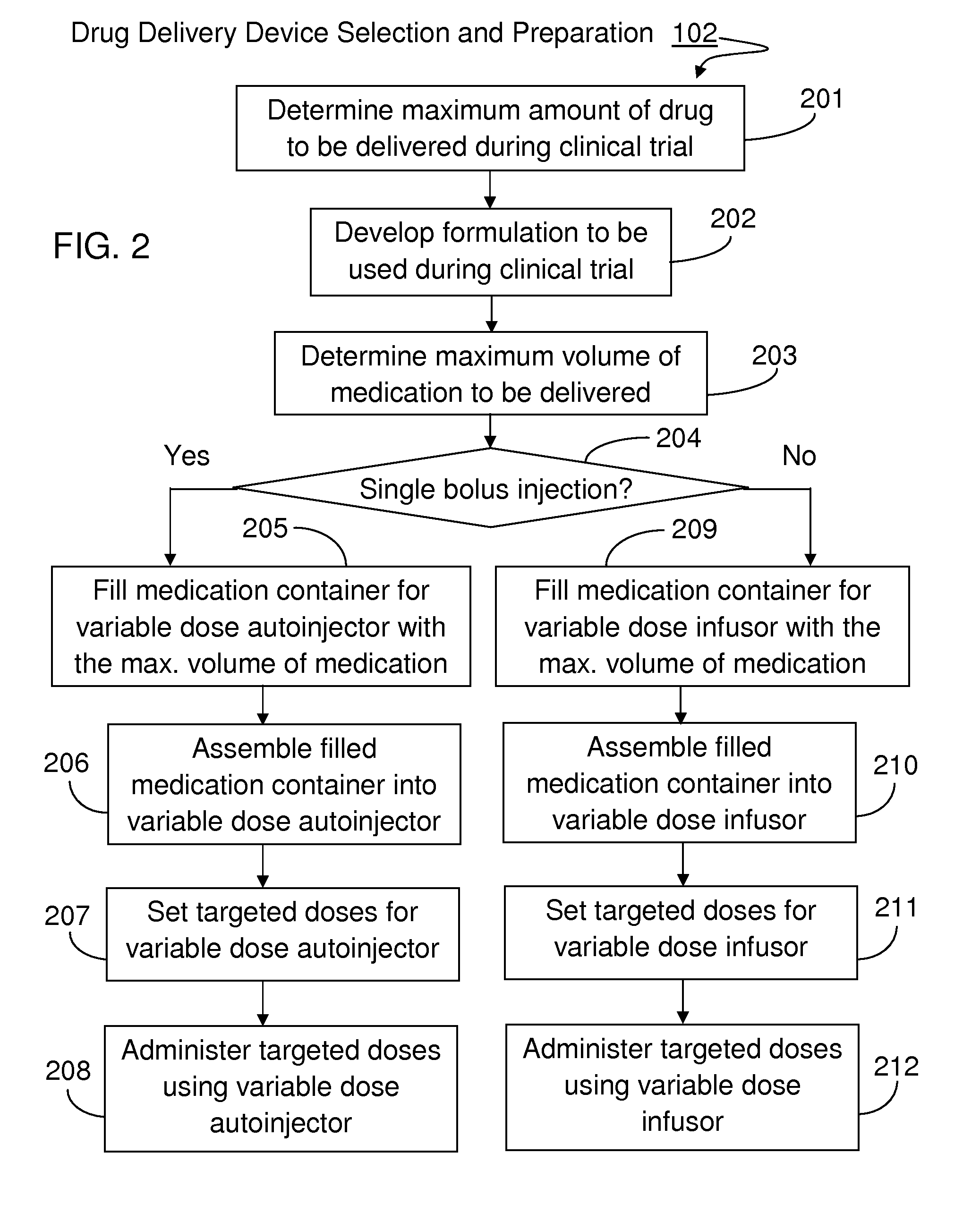
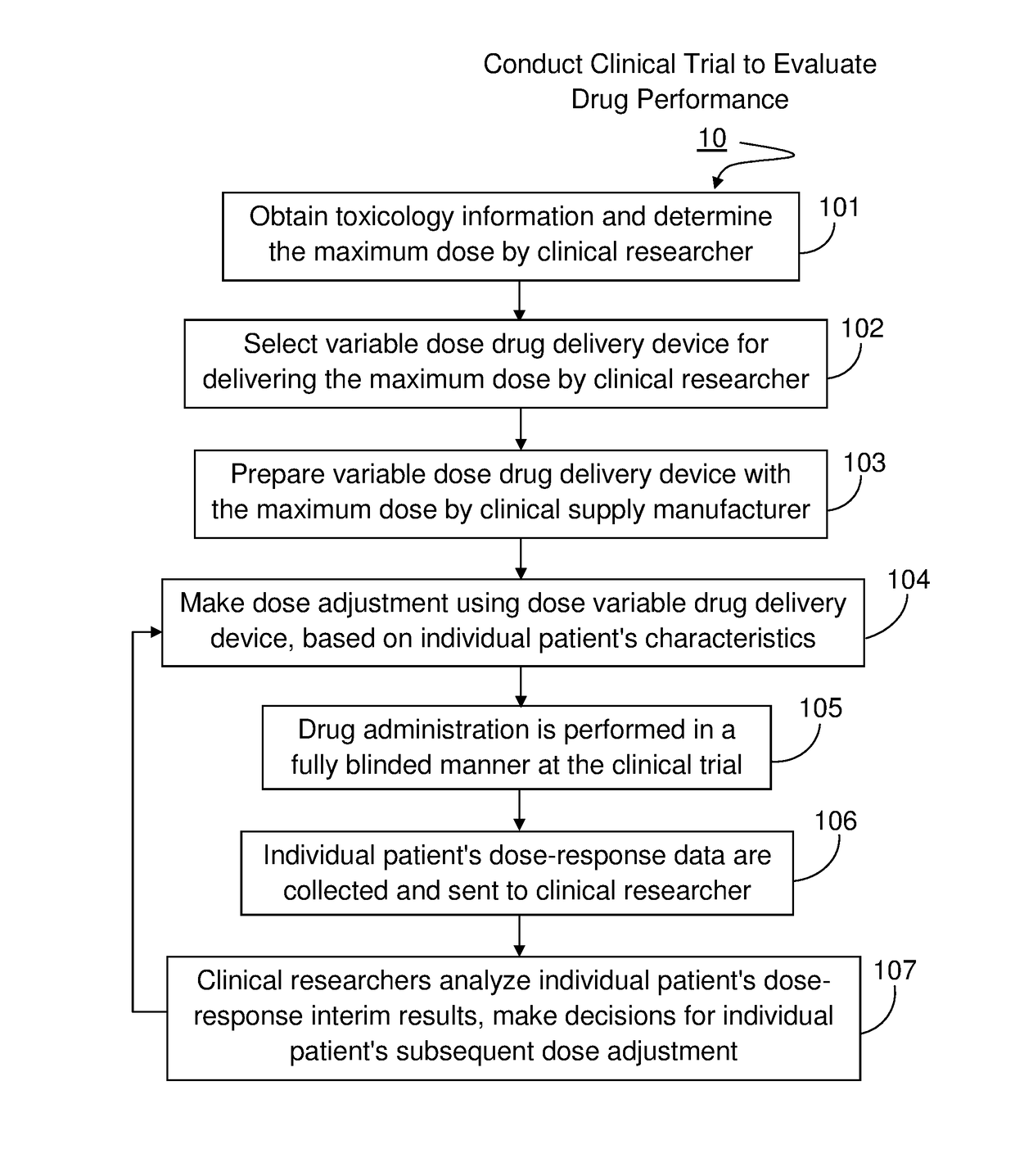
Method For Developing Parenteral Therapeutic Product With Drug Delivery Device Through Clinical Trial
InactiveUS20160092659A1Easy to useSimple preparation procedureAmpoule syringesData processing applicationsDose findingClinical trial
The method set out herein involves conducting clinical trial to develop parenteral therapeutic product with drug delivery device. The method comprises conducting a dose-finding clinical study using a variable dose drug delivery device to evaluate multiple clinical doses, wherein the variable dose drug delivery device is automatic, portable and for user self-administration. During the dose-finding clinical study, both the performance of the drug and the performance of the variable dose drug delivery device are evaluated.
Owner:WEI MIN
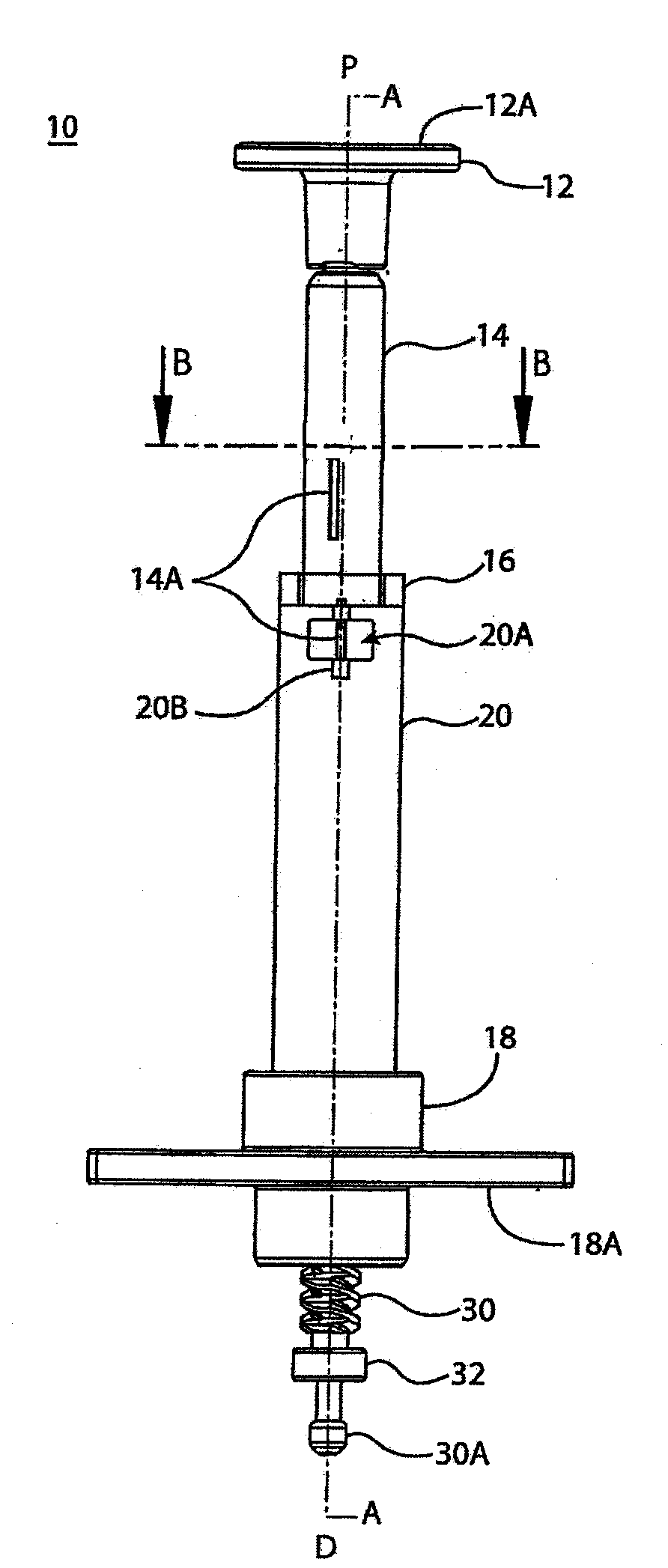
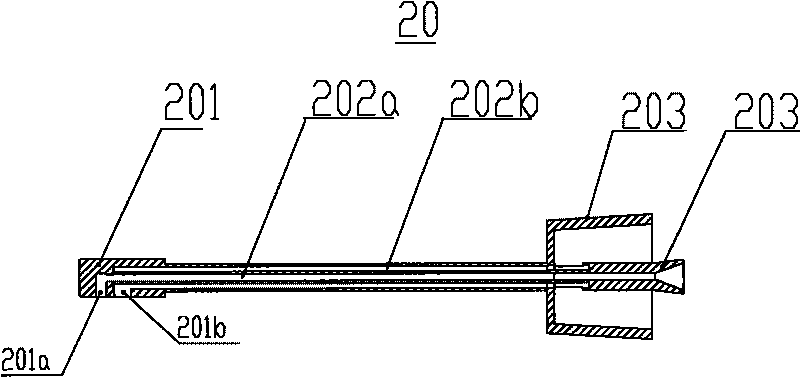
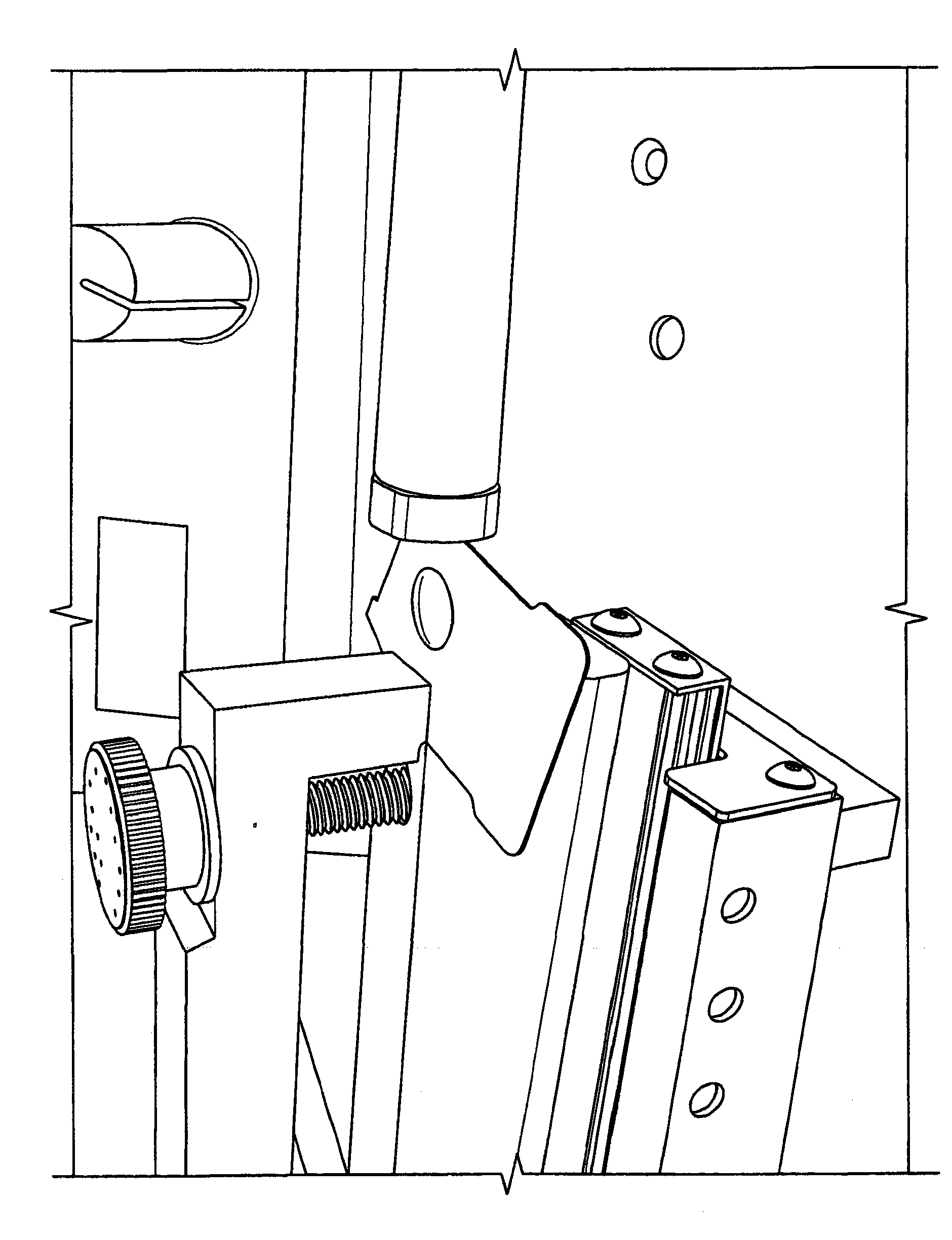
Accurate dose control mechanisms and drug delivery syringes
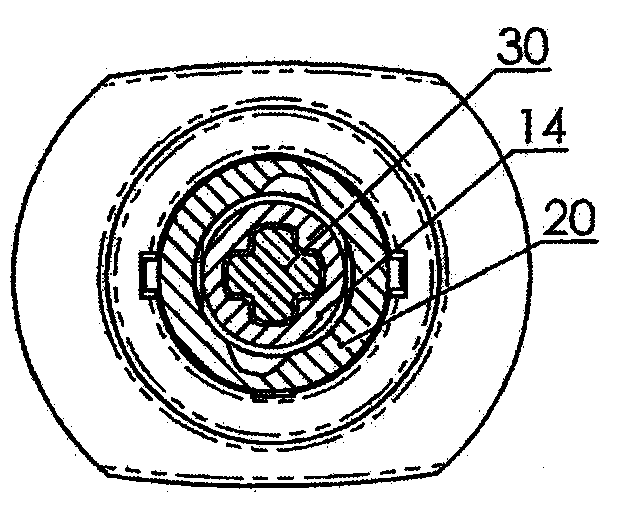
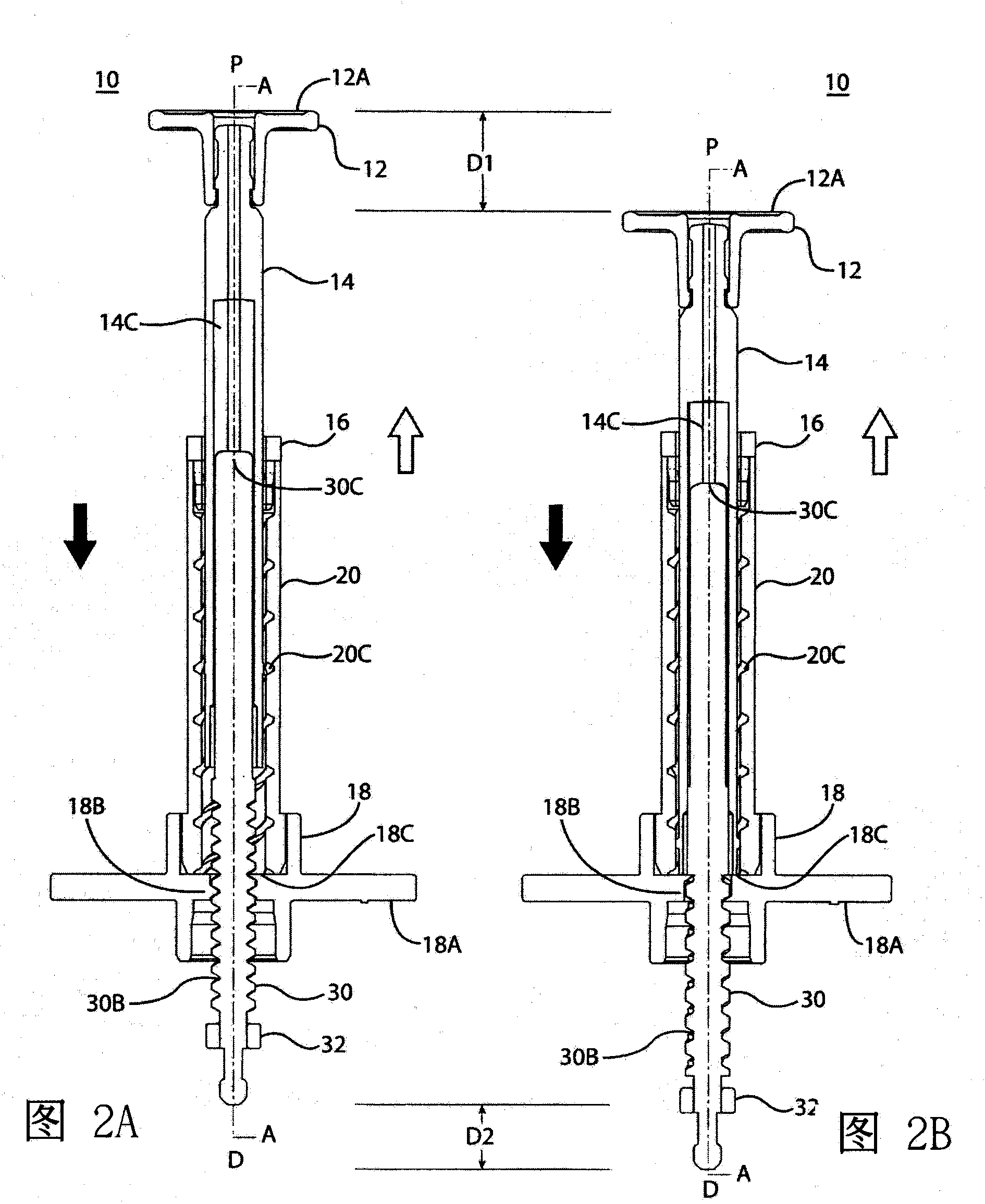
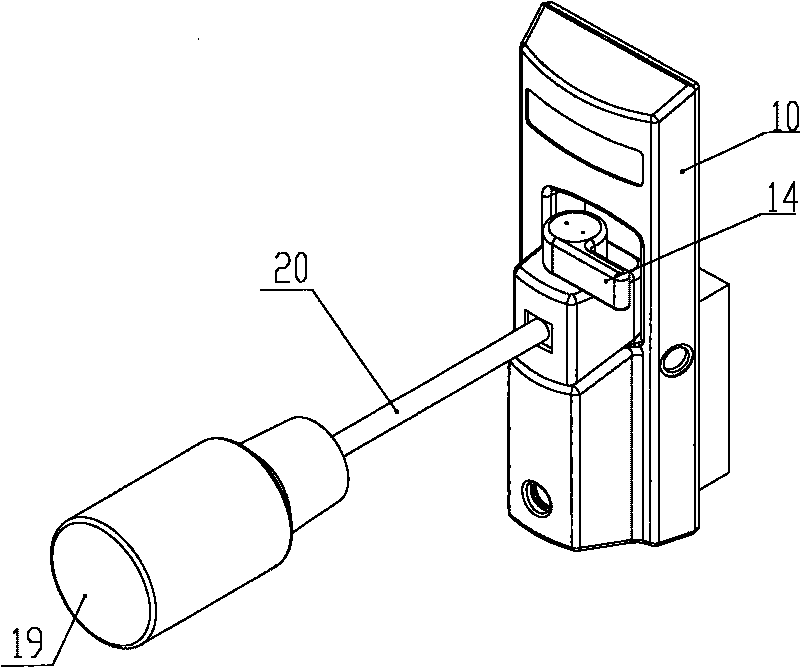
A dose control mechanism 10 for a syringe includes a plunger 14 having a coarse pitch screw 14B on its exterior surface, a housing 20 having a corresponding coarse pitch guide 20C along the interior surface of the housing 20, a screw 30 having a fine pitch screw 30B which interfaces with a fine pitch nut 18B of an adapter 18, wherein the plunger 14 has an internal annular space 14C within which screw 30 at least partially resides. An accurate dose drug delivery syringe includes such a dose control mechanism 10, a barrel 140, a plunger seal 136, and a barrel adapter assembly 150 having a barrel tip 152 and a needle 154. The syringe may be a fill-at-time-of-use syringe 100, a pre-filled syringe 200, or a safety syringe 300 having integrated needle retraction or needle sheathing safety features, or a combination thereof. Methods of assembly, manufacturing, and operation are similarly disclosed.
Owner:UNITRACT SYRINGE
Anesthetic vaporizer charging system
ActiveCN101757712AComposite applicationAchieving Zero LeakageRespiratorsAnesthesia vaporizerComposite application
The invention discloses an anesthetic vaporizer charging system, comprising an injection seat, a connecting seat and a charging device, wherein the injection seat is connected with the main body of the anesthetic vaporizer, and is provided with a dosing port; the connecting seat is hermetically arranged on the dosing port of the injection seat; and the charging device is arranged on the corresponding connecting seat to dose drugs for the dosing port. In the system, composite application in dosing mode for an anaesthesia machine can be realized through the connecting seat on the dosing port of the injection seat. In a plug-in adaptor charging mode, a compacting device can realize zero leakage in a charging process.
Owner:BEIJING AEONMED
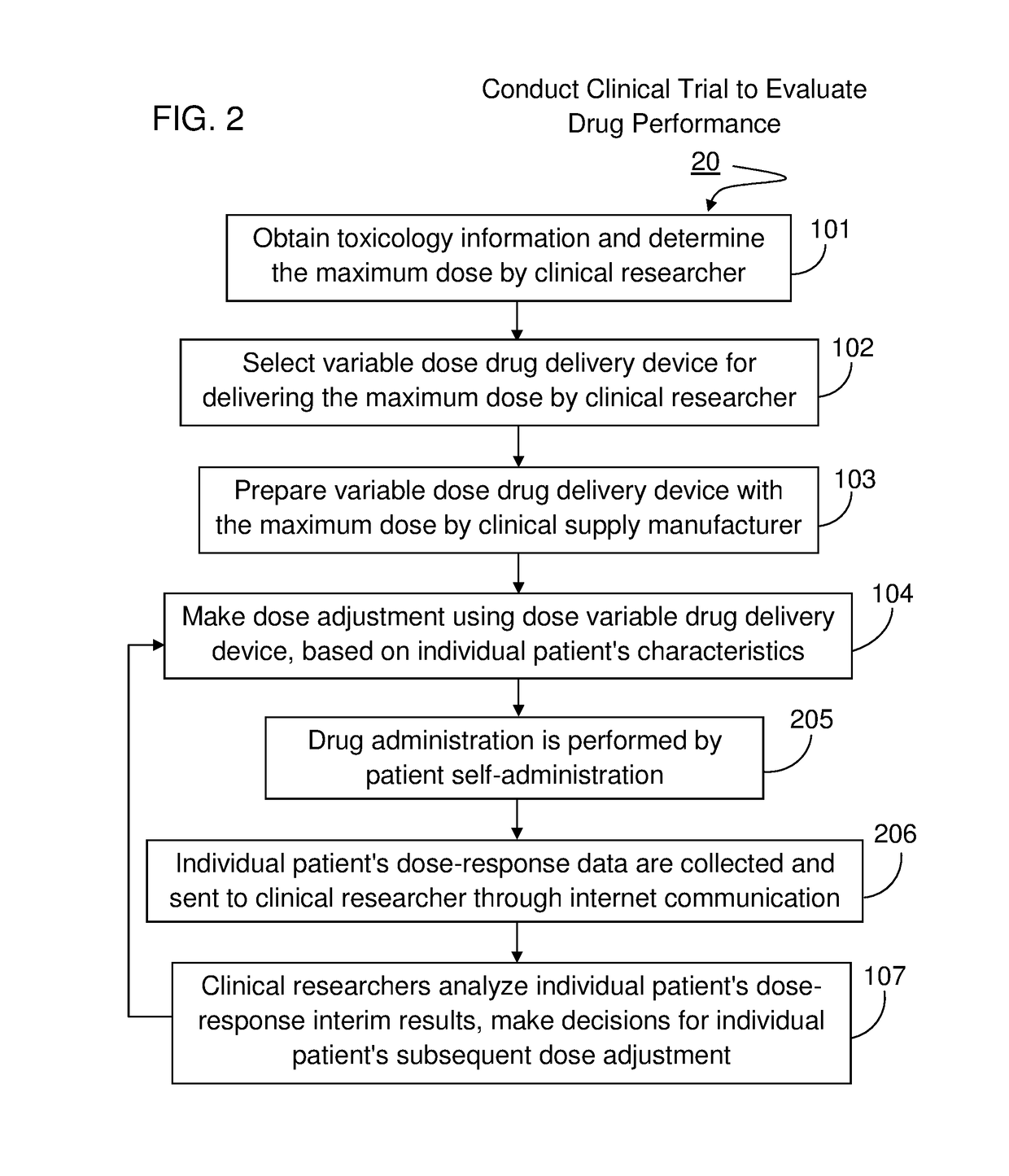
Method For Conducting Adaptive Clinical Trial With Drug Delivery Device
ActiveUS20170300665A1Efficient and cost-effectiveEfficient and cost-effective manufacturingDrug and medicationsComputer-assisted medical data acquisitionDosing drugsAdaptive clinical trial
The method set out herein involves conducting adaptive clinical trial to develop parenteral therapeutic product with variable dose drug delivery devices. The method comprises using a variable dose drug delivery device to respond modifications during the adaptive clinical trial, wherein the variable dose drug delivery device is able to deliver more than one dose level and is for delivering fluid formulation. Other methods set out herein involve using a variable dose drug delivery device equipped with radio frequency identification (RFID) or near field communication (NFC) technology to improve patient adherence to drug administration in the adaptive clinical trial.
Owner:WEI MIN
High-dispersity composition of low-dose drugs and preparation method of high-dispersity composition
ActiveCN108524454ANo residueHigh dissolution rateOrganic active ingredientsPharmaceutical product form changeAnalgesics drugsPsychosis drug
The invention discloses a high-dispersity composition of low-dose drugs and a preparation method of the high-dispersity composition, aiming to solve the problem that existing preparation methods for the low-dose drugs cannot meet requirements on content uniformity and influences of parameter change amplification in the production process on physicochemical properties of the low-dose drugs are remarkable. The high-dispersity composition and the preparation method thereof have the advantages that the low-dose drugs are prepared by the twin-screw extrusion technology for the first time; Vitamin Danalogue drugs, glucocorticoid drugs, antipsychotics, antipyretic and analgesic drugs and other low-dose drugs are screened as research objects; preparation of solid preparations through a twin-screwextruder is studied deeply; the content uniformity meets requirements of the pharmacopeia.
Owner:NANJING HERON PHARM CO LTD
Drug and food storage and distribution bottle
InactiveCN101700209AImprove work efficiencyReduce labor intensityPharmaceutical containersMedical packagingFood safetyDosing drugs
The invention provides a drug and food storage and distribution bottle comprising a cover and a body, wherein the cover is arranged on the body and is provided with a button; the body is provided with a drug dropping opening and is provided with a drug dropping mechanism at the bottom; and the drug dropping mechanism is driven to drop single-dose drugs from the drug dropping opening by pressing the button on the cover. The single-dose drug distribution bottle of the invention is provided with the unique single-dose drug dropping mechanism, so that users can take out the drugs or food in the bottle by single dose only by pressing the button on the cover. The bottle is simple and convenient in operation and can ensure the drugs or food which is put in first is taken out, thus ensuring the safety of the drugs and food, simultaneously increasing the working efficiency and reducing the labor intensity.
Owner:BEIJING OUMAI CENTURY SCI & TECH
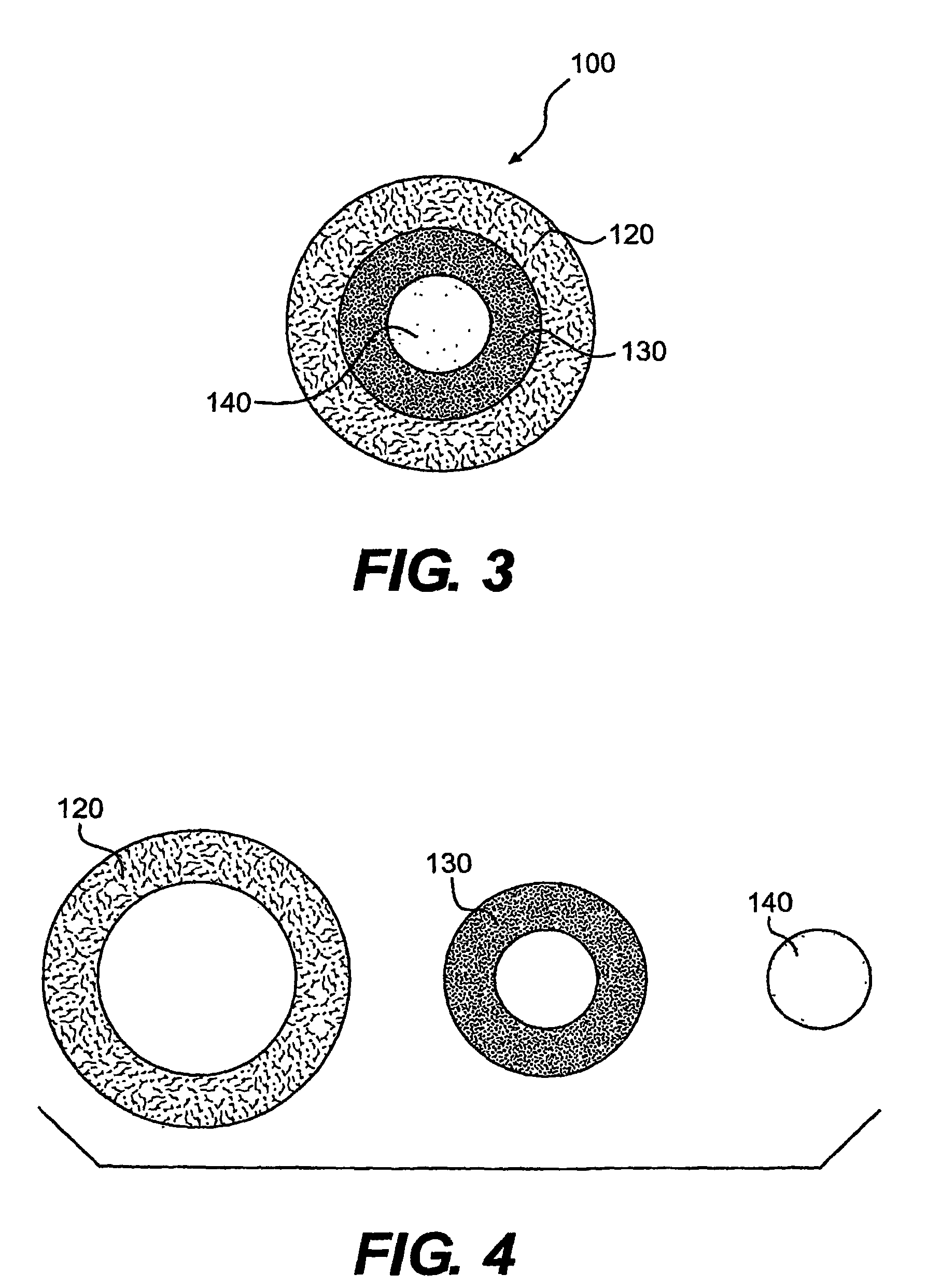
Drug delivery system using a solubilized gelatin shell composition and unit dose drug delivery using a special shape soft gelatin capsule
Disclosed is an improved drug delivery device for delivering a fill pharmaceutical composition. The drug delivery device comprises a soft gelatin capsule having a shell comprising gelatin and plasticizer wherein the shell is dissoluble upon dispersion into warm water. The invention also discloses snip-off or twist-off soft gelatin capsules offering unit dose convenience. The composition of the shell used in constructing the soft gelatin capsules comprises gelatin in the range of approximately 40% to 48% and a plasticizer ranging in amount from approximately 14% to 25%.
Owner:M S STRIDES
Powder conditioning of unit dose drug packages
Owner:NOVARTIS AG
Method for customized dispensing of variable dose drug combination products for individualizing of therapies
Owner:GLAXO SMITHKLINE LLC
Single-dose drug connected medicine bag and method
The invention discloses a single-dose drug connected medicine bag and a method. The connected medicine bag is composed of a bag body, a zipper and sealing and cutting lines; the number of the sealing and cutting lines is plural, and the bag body is evenly divided into a plurality of single body medicine bags by the plurality of sealing and cutting lines; the zipper is arranged at the upper end of the bag body in the transverse direction; and an interval is arranged between the top end of each sealing and cutting line and the zipper. The medicine containing method for the connected medicine bag includes that the connected medicine bag is placed on a sealing and packaging machine; the position of a bag opening mechanism is adjusted and made to be located at the position of the zipper; medicine use labels of a patient are printed by a printer; the medicine use labels adhere to the single body medicine bags by an adhering mechanism; a conveying device conveys the single body medicine bags where the medicine use labels adhere to the position of the bag opening mechanism; the medicine bags are opened by the bag opening mechanism, and a funnel stretch into each bag opening for containing medicine; the medicine bags are conveyed to the position of a rolling wheel, and the rolling wheel extrudes the zipper to seal the medicine bags; and the above operation is repeated till medicine containing is completely completed. According to the single-dose drug connected medicine bag and the method, single-dose medicine containing can be performed according to the actual treatment course, and the beneficial effects that pollution is avoided, the automation degree is high, and medicine dispensing is accurate and efficient are achieved.
Owner:BEIJING REDMAPLE INTELLIGENT CONTROL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com