High-dispersity composition of low-dose drugs and preparation method of high-dispersity composition
A low-dose, composition technology, applied in the low-dose pharmaceutical composition and its preparation, the low-dose pharmaceutical composition and its preparation field, can solve the problems such as the application of twin-screw extrusion technology that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
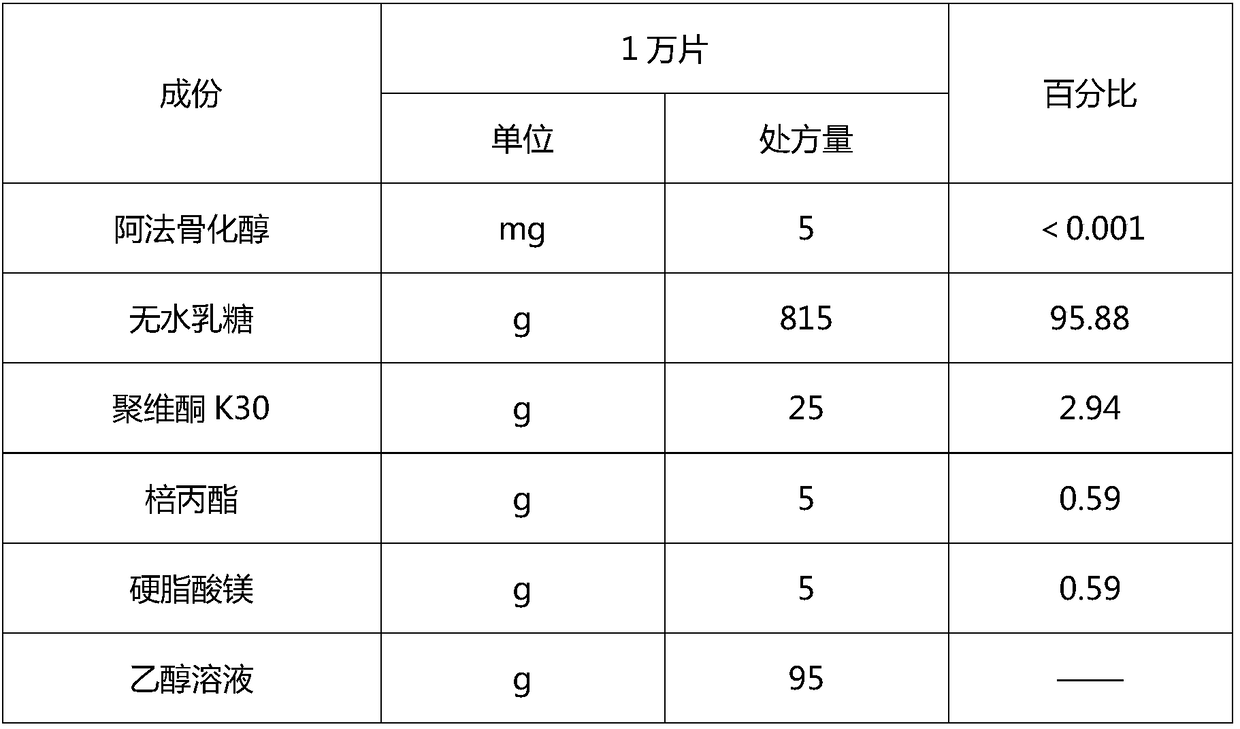
[0083] Preparation of Embodiment 1 Alfacalcidol Tablets, Small Test Process
[0084] 1. Prescription
[0085]
[0086] 2. The preparation method specifically comprises the following steps:
[0087] 1) dissolving the crude drug alfacalcidol in absolute ethanol to make a solution;
[0088] 2) Weigh the prescribed amount of anhydrous lactose, povidone, and propyl gallate in a wet mixing granulator, and the mixing time is greater than or equal to 5 minutes to make dry powder;
[0089] 3) Add the dry powder obtained in the above step 2) into the solid feeder, and after the solution obtained in the above step 1) is connected to the extruder through a low-pulse peristaltic pump, control the granulation temperature of the twin-screw extruder at 20-25 Between ℃, adjust the feeding speed of dry powder to 1.0-2.0kg / hr, the speed of peristaltic pump to 2rpm-4rpm, and the speed of twin-screw to 100rpm-200rpm, and the above-mentioned dry powder and solution enter the twin-screw extrude...
Embodiment 2
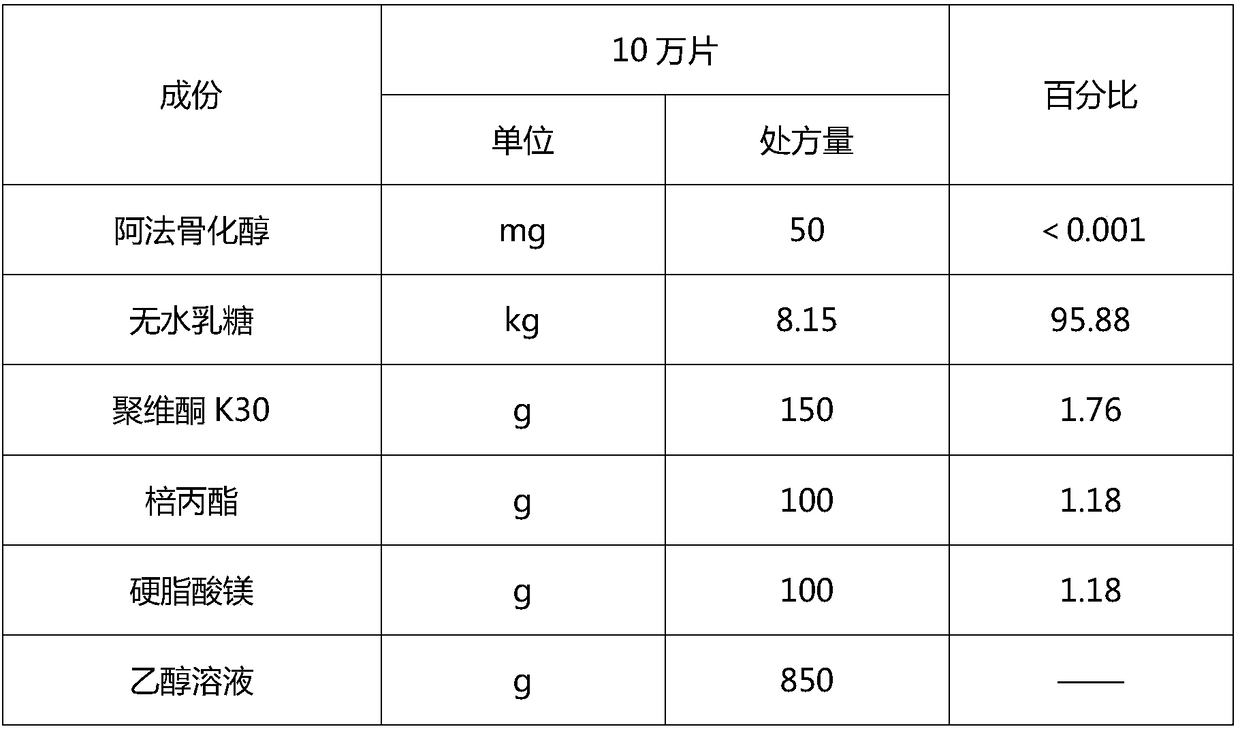
[0093] Example 2 Preparation of Alfacalcidol Tablets, Pilot Test Process
[0094] 1. Prescription
[0095]
[0096] 2. The preparation method specifically comprises the following steps:
[0097] 1) dissolving the crude drug alfacalcidol in absolute ethanol to make a solution;
[0098] 2) Weigh the prescribed amount of anhydrous lactose, povidone, and propyl gallate in a wet mixing granulator, and the mixing time is greater than or equal to 5 minutes to make dry powder;
[0099] 3) Add the dry powder obtained in the above step 2) into the solid feeder, and after the solution obtained in the above step 1) is connected to the extruder through a low-pulse peristaltic pump, control the granulation temperature of the twin-screw extruder at 20-25 Between ℃, adjust the feeding speed of dry powder to 2.0-3.0kg / hr, the speed of peristaltic pump to 4rpm-8rpm, and the speed of twin-screw to 300rpm-400rpm, and the above-mentioned dry powder and solution enter the twin-screw extruder w...
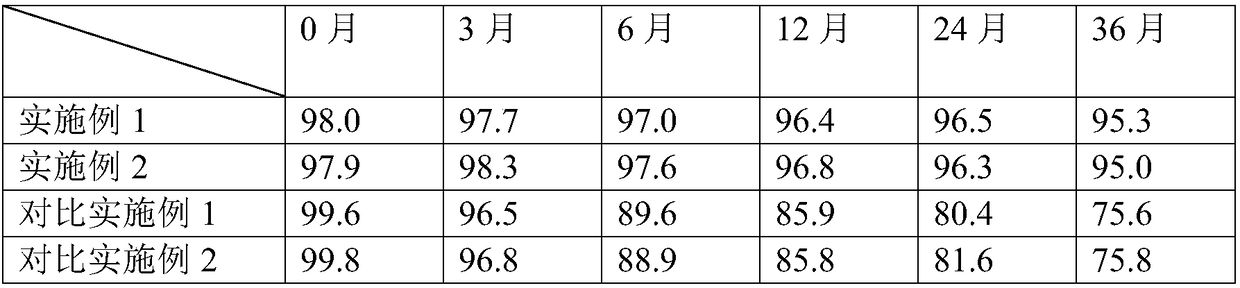
Embodiment 3 comparative Embodiment 1
[0103] Example 3 Comparative Example 1 CN104739793A discloses a kind of alfacalcidol tablet and its preparation method, adopts traditional wet granulation, according to the preparation method of Example 1 in the text, uses the same prescription of the present invention, obtains comparative example 1 Alpha tablets. The process description is as follows: Weigh the raw and auxiliary materials of the prescription amount, pass through 80 mesh sieves respectively for standby; configure the adhesive solution; Soft material; after granulating with a 40-mesh sieve, dry at 50°C for 30 minutes, pass through a 20-mesh sieve for granulation, add magnesium stearate, mix evenly, and press into tablets.
[0104] Example 4 Comparative Example 2 CN1196677A discloses a preparation method of a very low-dose solid dosage form of medicine, using high-speed shear mixing granulation technology, according to the preparation method in the text, using the same prescription of the present invention, to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com