Formulations of human tissue kallikrein-1 for parenteral delivery and related methods
a technology of human tissue and kallikrein, which is applied in the direction of drug composition, peptide/protein ingredient, metabolic disorder, etc., can solve the problems of many complications, achieve the effects of improving pharmacokinetics, improving bioavailability, and improving systemic pharmacokinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0117]A cDNA coding for pre-pro-human KLK1, the 262 amino acid residue sequence depicted in SEQ ID NO:2, was purchased from OriGene™ (Rockville, Md., USA). The KLK1 cDNA (Catalogue No. SC122623) is a human cDNA open reading frame clone, cloned into the multi-cloning site of OriGene's pCMV6-XL5 vector, between a cytomegalovirus (CMV) promoter to control transcription of cDNA coding for pre-pro-human KLK1 and a polyadenylation signal. This KLK1 clone was sequenced and, using translation software, translated to reveal SEQ ID NO:2. This sequence differed at 2 amino acid residues from the human KLK1 sequence in GenBank as Ref No. NP—002248.1 (SEQ ID NO:1). As depicted in SEQ ID NO:2, single nucleotide polymorphisms (SNP's) resulted in an E to Q change at amino acid residue 145 of 262, and an A to V change at amino acid position 188 of 262. All subsequent experiments were performed with KLK1 having the amino acid sequence in SEQ ID NO:2.
[0118]The human KLK1 cDNA in the pCMV6-XL5 was trans...
example 2
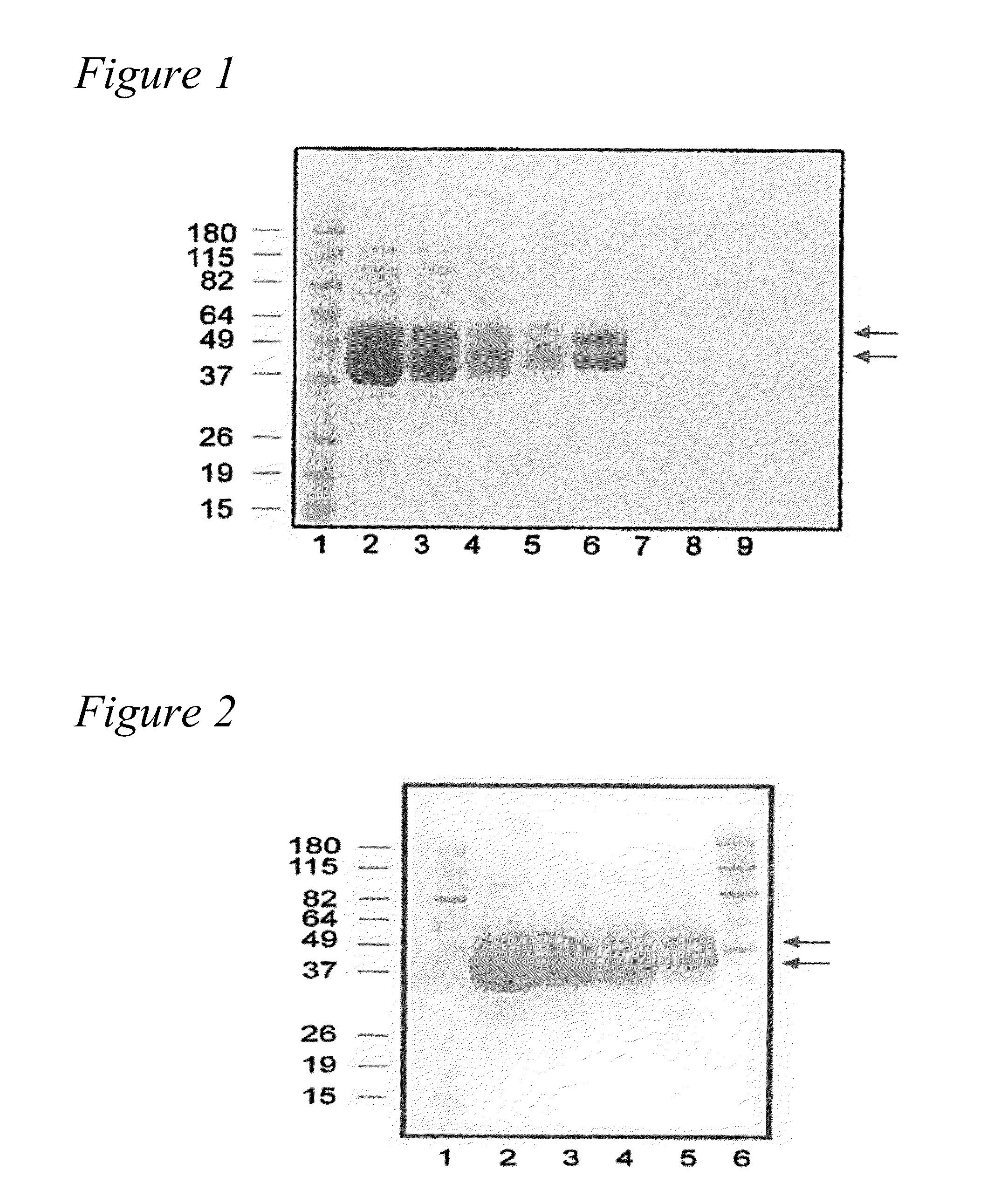
[0121]The purified recombinant human KLK1 contained approximately 30% carbohydrate content based on the molecular weight estimated by sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis (see FIG. 1). KLK1 from CHO cells appears as a band having an apparent molecular weight of ˜40 to 49 kDa; such a broad band may result from different glycosylation foams of KLK1 secreted by CHO cells. For KLK1 expressed in 293 cells, two bands appeared on the SDS-PAGE gel at approximately 40 kDa and 45 kDa. The identity of the bands as human KLK1 was confirmed by Western blot analysis using mouse polyclonal antibody raised against a full-length human KLK1 protein (Catalog # H00003816-B01P, KLK1 purified MaxPab mouse polyclonal antibody (B01P), Abnova Corporation, Walnut, Calif., USA) (see FIG. 2). The Western blot confirms the results of the SDS-PAGE gel, in that recombinant human KLK1 from CHO cells appears as a band having an apparent molecular weight of ˜40 to 49 kDa, and KLK1 express...
example 3
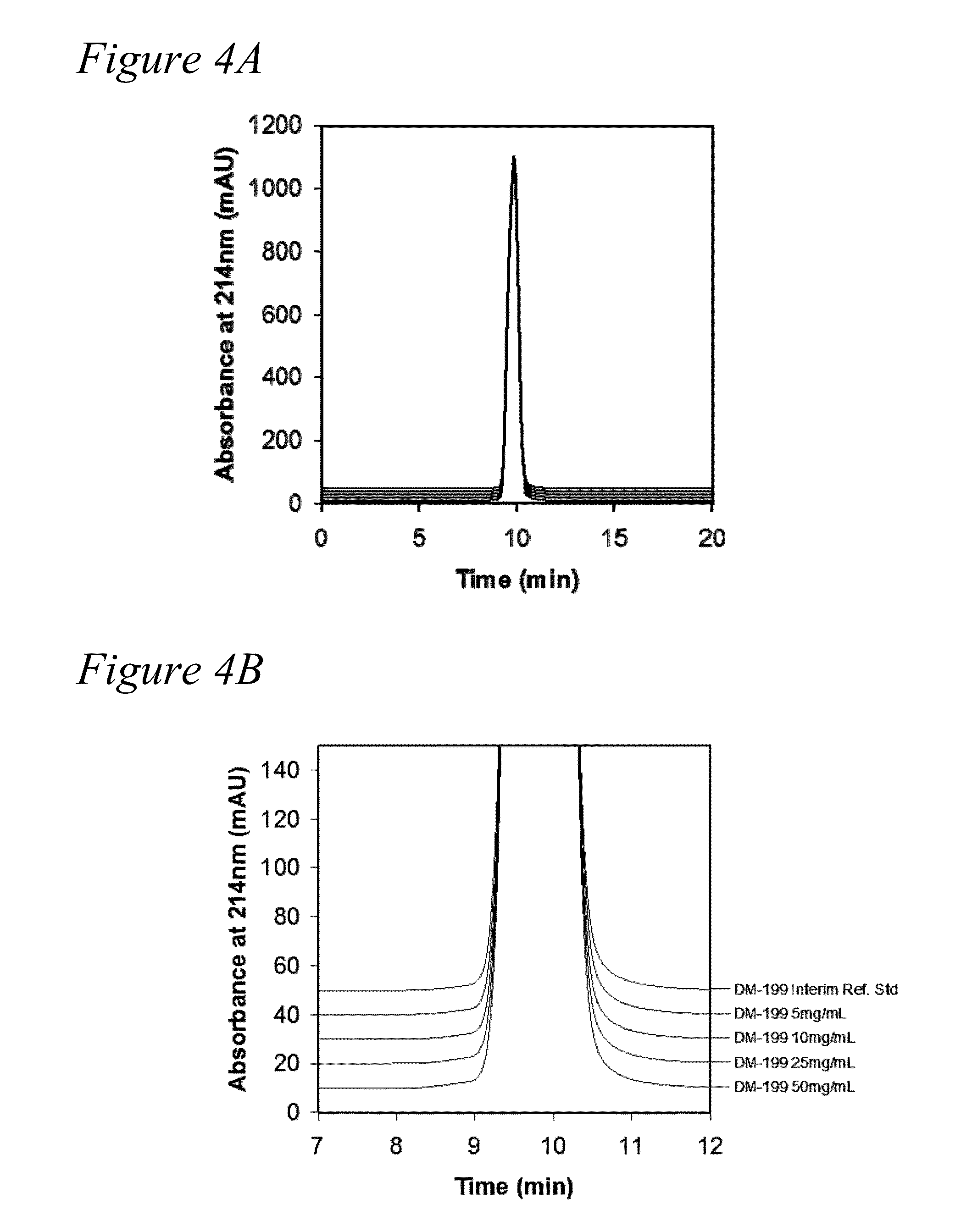
[0126]Generation of concentrated formulation of rhKLK1. The following example determined that rhKLK1 could be concentrated to at least 50 mg / mL, and that the resulting high concentration formulation would be stable and not aggregate.
[0127]The rhKLK1 having at least 4 moles sialic acid per mole protein was formulated into formulations having 5 mg / mL, 10 mg / mL, 25 mg / mL and 50 mg / mL Bulk rhKLK1 was concentrated using VIVASPIN® 500 Centrifugal Concentrator 3,000 MWCO PES membrane. The protein concentration of rhKLK1 was determined by absorbance at 280 nm and the samples were close to the target concentration (5.0 mg / mL, 9.9 mg / mL, 26.2 mg / mL and 50.3 mg / mL). Following concentration, the samples were sterile filtered through a 0.2 μm filter. The rhKLK1 samples at the four different concentrations were filled into Schott borosilicate 3 cc, 13 mm serum vials at a fill volume of 0.5 mL, and the vials were sealed with a Daiko 13 mm injection stopper, Flourotec Plus, B2-40 rubber stopper. Th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com