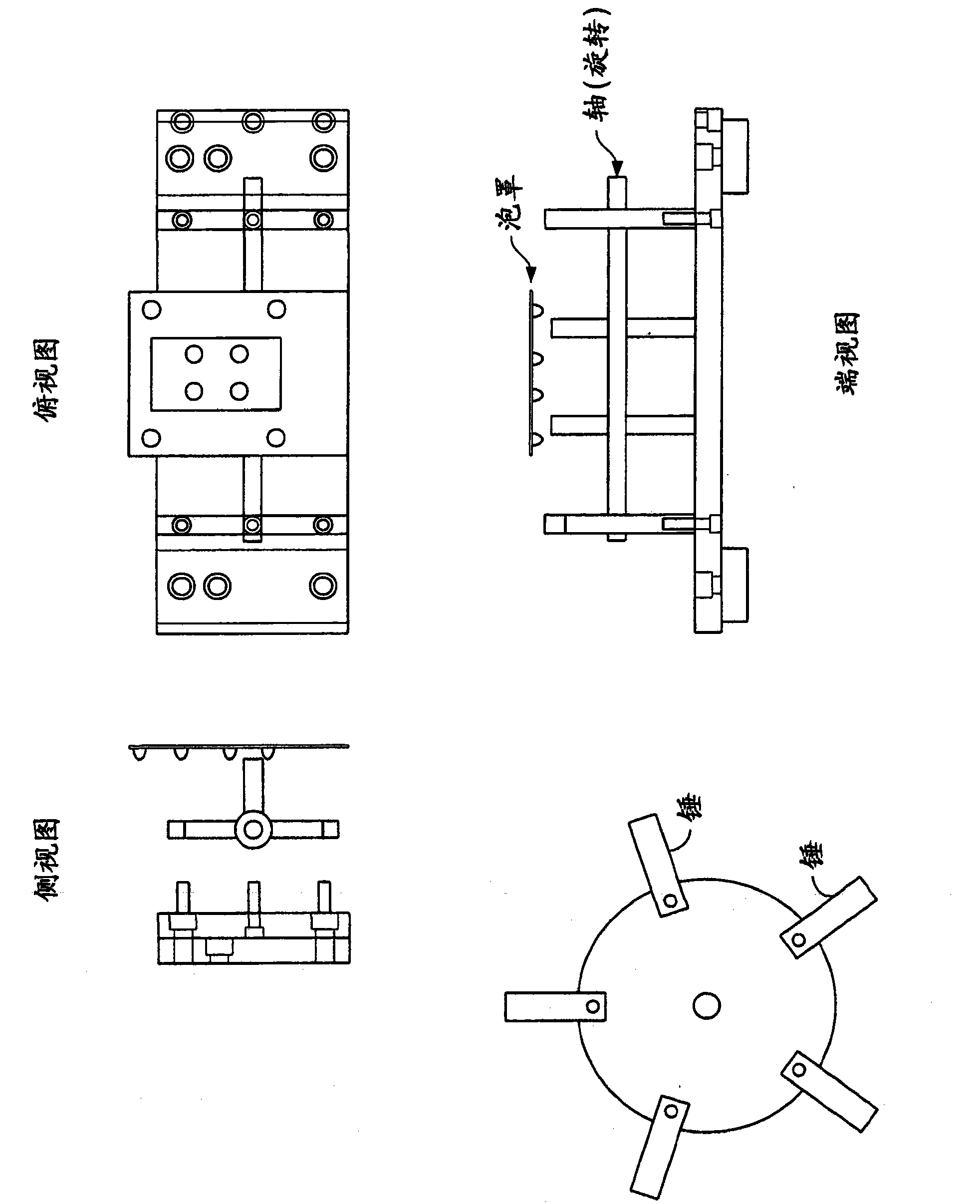
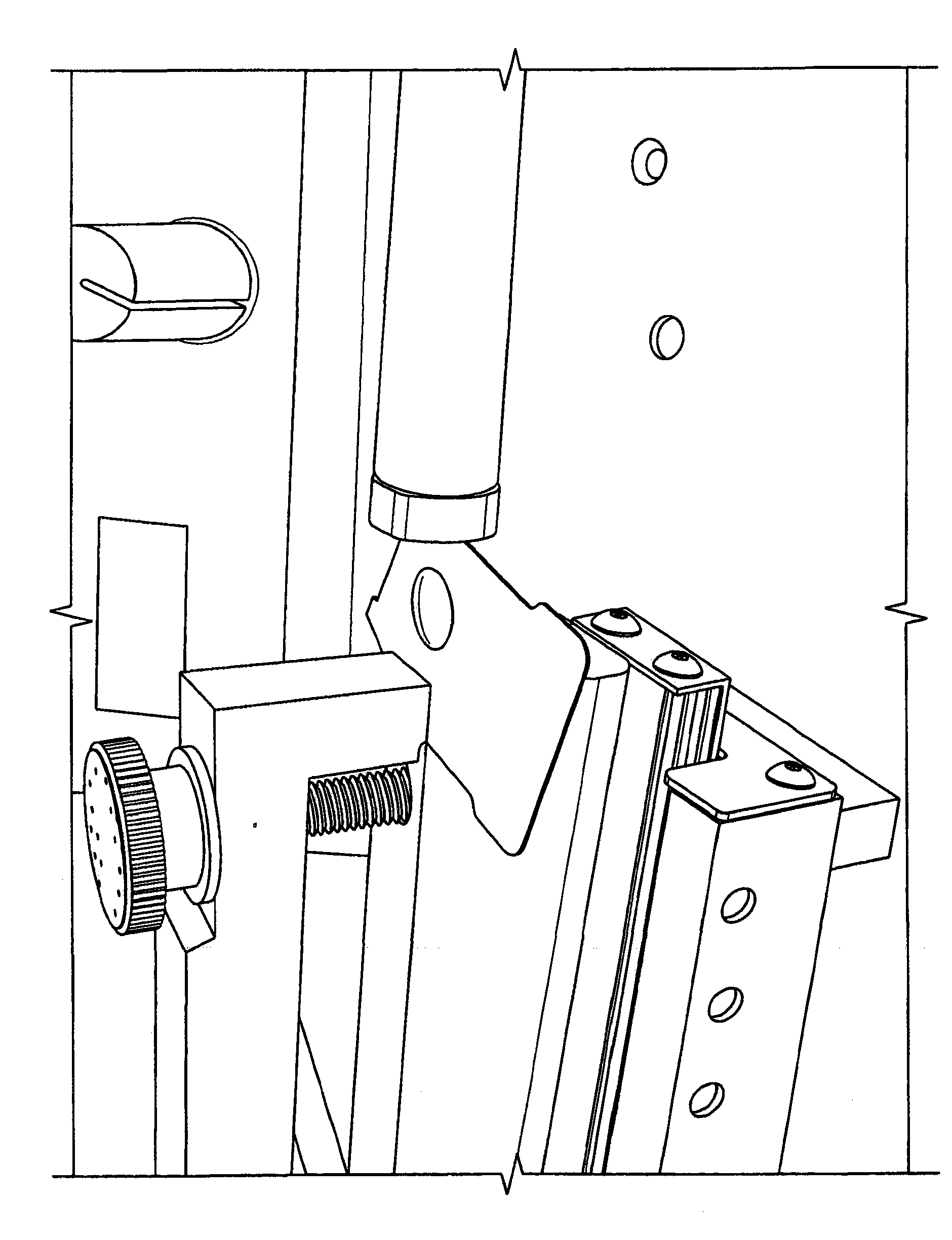
Powder conditioning of unit dose drug packages
A unit dose and drug technology, applied in the direction of drug equipment, packaging, transportation and packaging, etc., can solve the problem of different doses of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016] It must be noted that, as used in this specification, singular forms include plural referents unless the context clearly dictates otherwise.
[0017] In describing and claiming the present invention, the following terms are used according to the definitions set out below.
[0018] definition
[0019] Unless otherwise stated, the terms used herein are defined as follows. Unless explicitly defined herein, standard terms are given their ordinary and conventional meanings as understood by those of ordinary skill in the art.
[0020] The term "conditioning" is used to describe the process of causing a powder to disperse more uniformly, exhibiting less agglomeration than an unconditioned powder. "Deagglomeration" is used interchangeably to refer to conditioning.
[0021] A composition "suitable for pulmonary delivery" refers to a composition that is capable of being aerosolized and inhaled by an individual such that some of the aerosolized particles reach the lungs (eg, ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com