sybr Green I detection method of hla-b*1502 gene and its special primers and kits
An HLA-B, detection method technology, applied in DNA/RNA fragments, recombinant DNA technology, fluorescence/phosphorescence, etc., can solve the problems of low specificity and sensitivity, time-consuming and labor-intensive, and achieve wide applicability and simple operation procedures. , the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Design and screening of primers for SYBR Green I detection of HLA-B*1502 gene
[0040] Referring to the full sequence of the HLA-B*1502 gene included in GenBank, the 2nd and 3rd exons of the HLA-B*1502 gene were selected to design synthetic primers. In primer design, 2 or less degenerate bases are allowed at the same variable site.
[0041] In the screening process of primers, it is necessary to select primers with higher PCR amplification efficiency and SYBR Green I dye binding efficiency in the target conserved region (the PCR primers used are synthesized by Shanghai Invitrogen Company, and the primers require PAGE purification. The primers are dry powder when they arrive. , after reconstitution with sterile water, the dry powder is assayed, and the primers are stored at 0.4um / ul for later use). For the HLA-B*1502 gene, 2 sets of upstream and downstream primers (SYBR-1 and SYBR-2) were designed, and the upstream and downstream primers for detecting beta-ac...
Embodiment 2
[0055] Embodiment 2, the optimization of the reaction system that carries out SYBR Green I detection to HLA-B*1502 gene
[0056] 1. Optimization of Primer Consumption
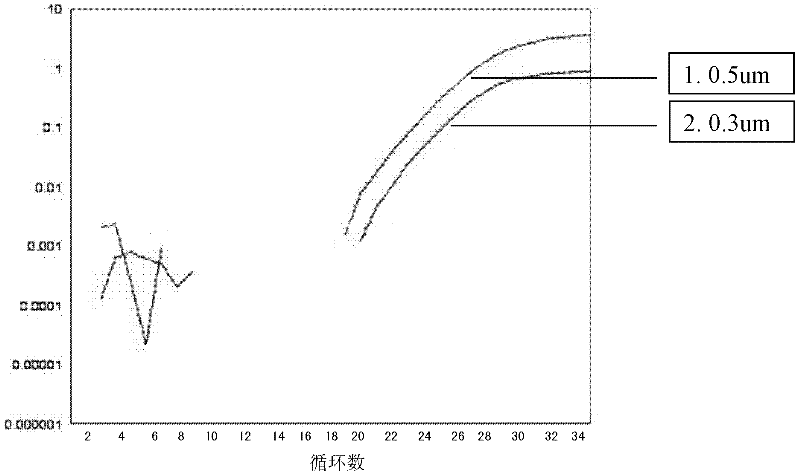
[0057] Primer concentration is a key factor affecting PCR reactions. If the primer concentration is low, the reaction will be incomplete. If there are too many primers, the possibility of mismatches and non-specific products increases. The amount of primers for the upstream and downstream were chosen to be the same. Primer concentrations range from 0.1-1um. In the experiment, three different primer concentrations of 0.3um, 0.5um, and 1um were used to detect SYBR Green I against the same template (peripheral blood genomic DNA of patients carrying the HLA-B*1502 gene). The conditions are the same as in Example 1.
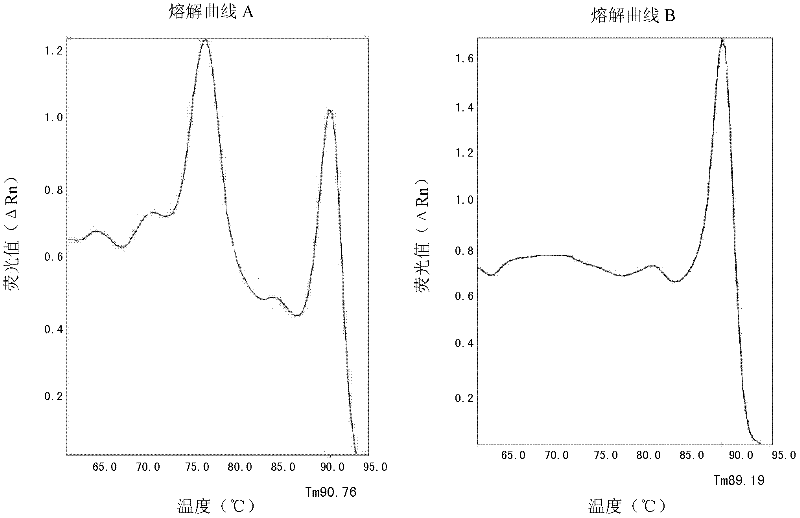
[0058] The result is as Figure 2A-Figure 2D as shown ( Figure 2A It is the SYBR Green I amplification curve under the primer concentration of 0.3um and 0.5um (curve 1 is 0.5um, curve 2 is 0.3u...
Embodiment 3
[0063] The SYBR Green I detection of embodiment 3, HLA-B*1502 gene
[0064] 1. Construction of the recombinant plasmid p-HLA-B*1502 carrying the HLA-B*1502 gene
[0065] The HLA-B*1502 gene sequence was synthesized by Shanghai Yingjie Company, and cloned into the pGEM-T Easy vector (purchased from Promega Company) between the NoT I and Eco RI restriction sites of the vector multiple cloning site to obtain the recombinant vector p- HLA-B*1502.
[0066] 2. Detection of 10 clinical samples by SYBR Green I method of HLA-B*1502 gene
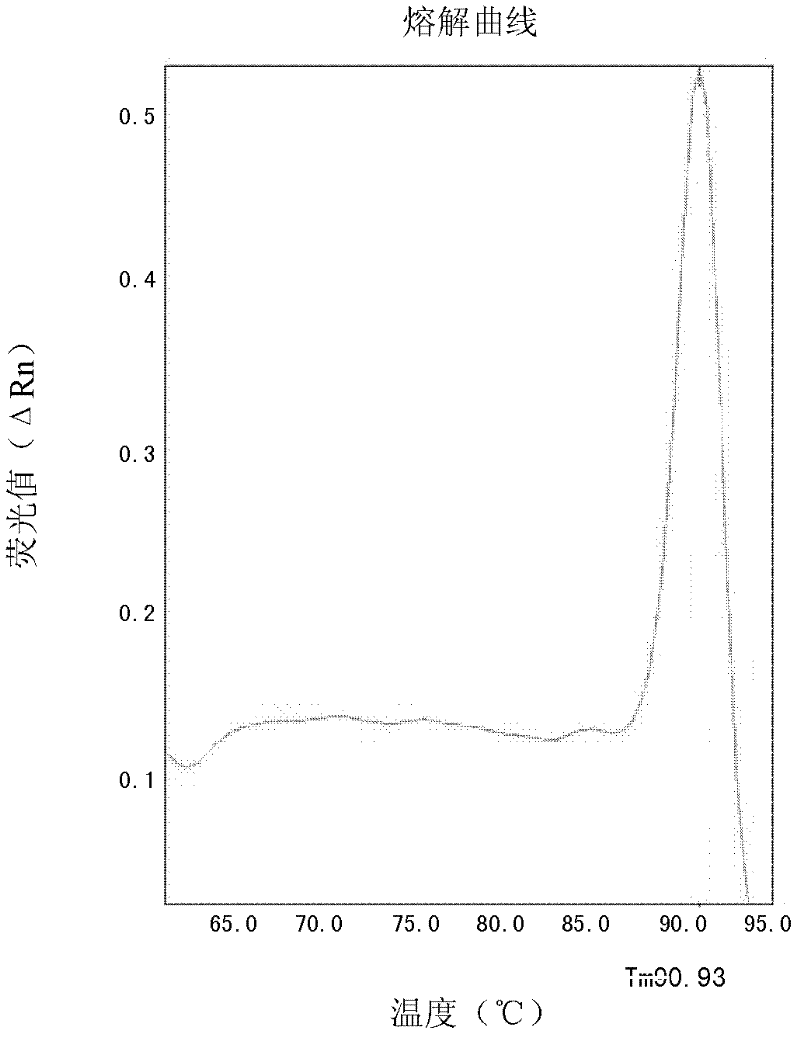
[0067] The peripheral blood genomic DNA of 5 confirmed patients carrying the HLA-B*1502 gene and the peripheral blood genomic DNA of 5 patients without the HLA-B*1502 gene were used as templates (with p-HLA-B*1502 as the positive control, With sterile water as a negative control), the SYBR Green I method detection of the HLA-B*1502 gene was carried out with the SYBR-2 primers in Example 1 and the beta-actin internal reference gene primers. Each sam...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com