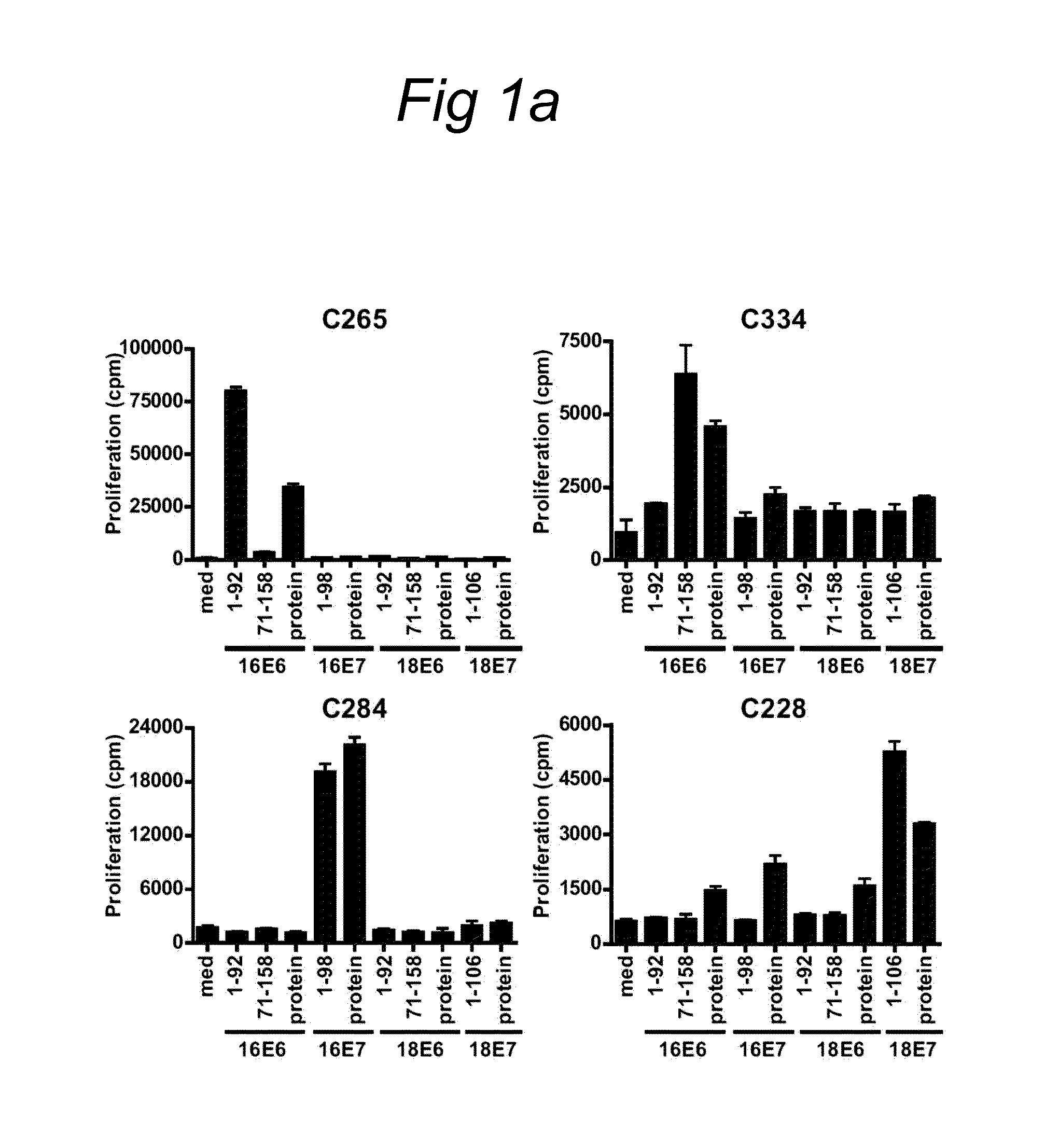
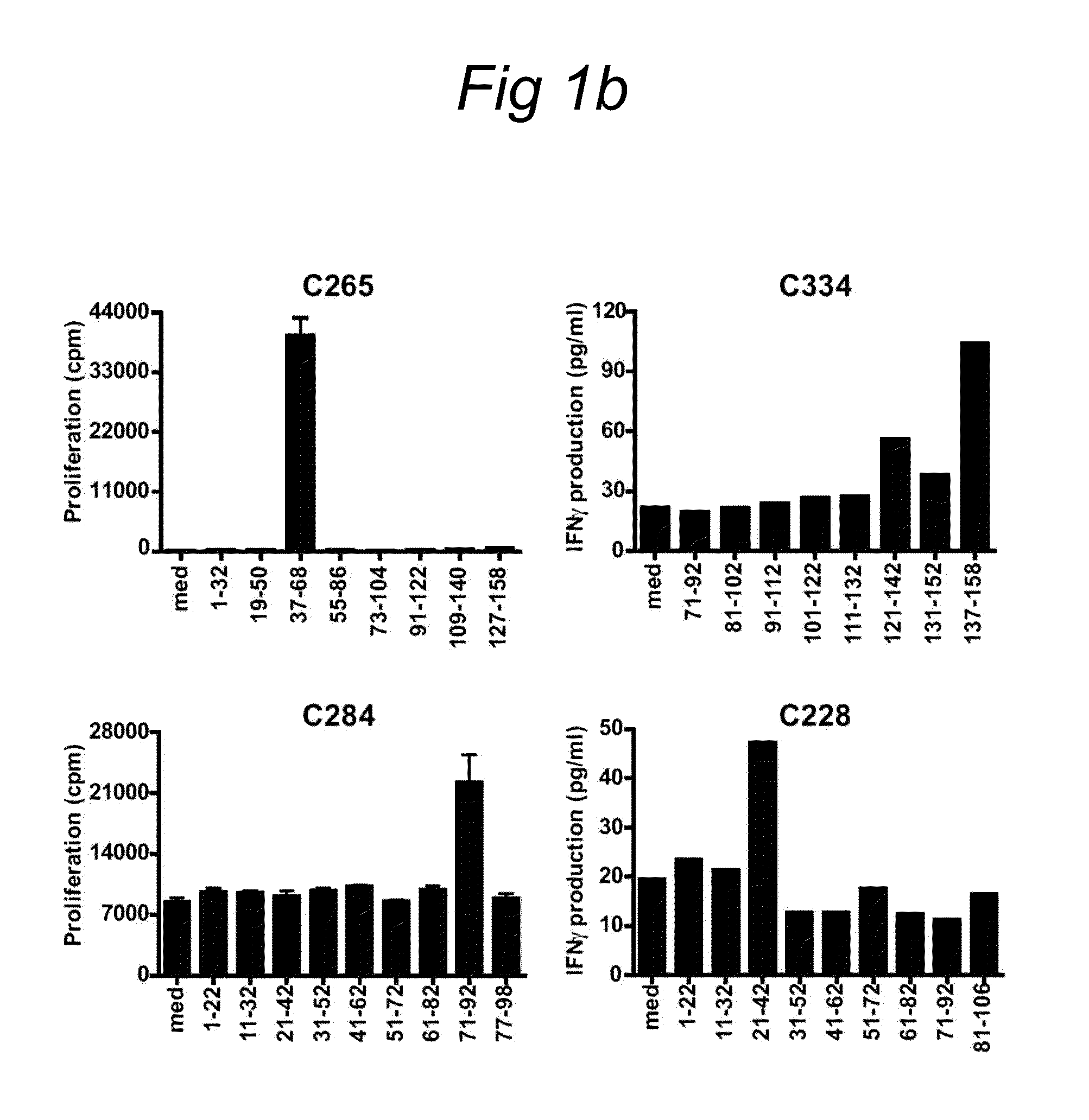
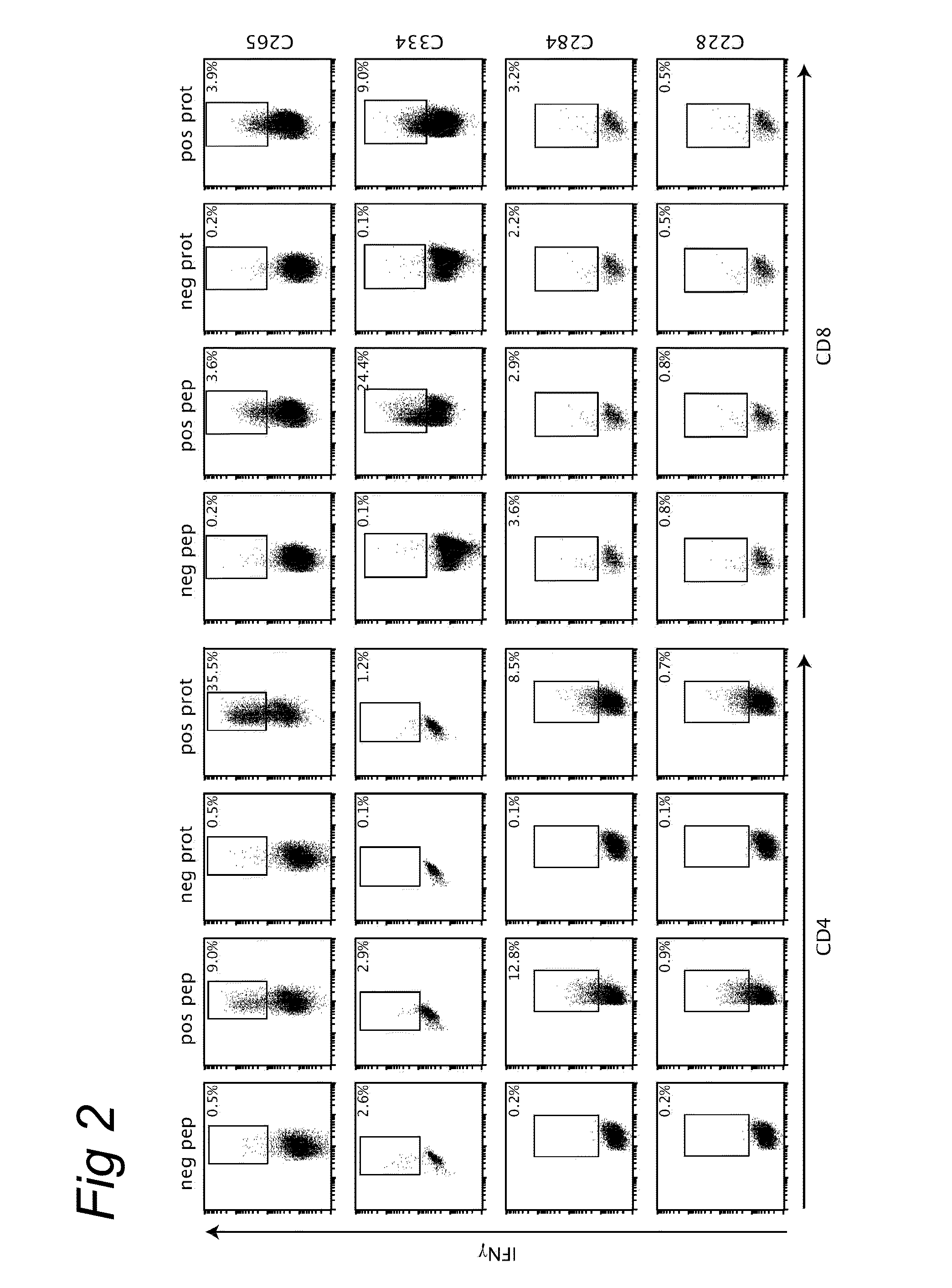
HPV epitopes targeted by T cells infiltrating cervical malignancies for use in vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification and Characterization of Novel HPV Epitopes
1. Methods
[0075]1.1 Subjects
[0076]Women presenting with histologically proven cervical neoplasia at the department of Gynaecology of the Leiden University Medical Centre and Leyenburg Hospital the Hague were enrolled in the CIRCLE study, which investigates cellular immunity against HPV16-positive cervical lesions after providing informed consent. The study design was approved by the Medical Ethical Committees of both hospitals. The subjects were tested for HPV status using HPV16 and HPV18 specific primers on DNA isolated from surgical resection specimens (Claas et al. 1989). Peripheral blood mononuclear cells (PBMC) for HLA-restriction analysis were obtained from HLA-typed anonymous healthy blood donors after informed consent.
[0077]1.2 Antigens
[0078]A set of overlapping peptides spanning both HPV16 and HPV18 E6 and E7 protein were used for T cell stimulation assays. HPV16 and HPV18 E6 and E7 consisted of 22-mers overlapping 12...
example 2
Intradermal Administration of a Peptide
Materials and Methods
[0101]Study Design
[0102]A cross-sectional pilot study to analyse HPV16 E2-, E6-, and E7-specific T-cell responses as measured by intradermal injection of pools of clinical grade HPV16 peptides in the upper arm was performed in patients with HPV-related disorders of the cervix and in healthy individuals. Since a delayed type hypersensitivity reaction represents a memory T-cell response, there was no prerequisite for HPV16-positivity at the time of analysis.
[0103]Subjects
[0104]A group of nineteen healthy individuals (HD) participated in this study after providing informed consent. The group of healthy individuals displayed a median age of 31 years old (range, 20-51 years) and was comprised of 80% women and 20% males. Peripheral blood mononuclear cells (PBMCs) were obtained from all subjects immediately before administration of the skin test. The late appearance of positive skin tests in healthy individuals resulted in the iso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com