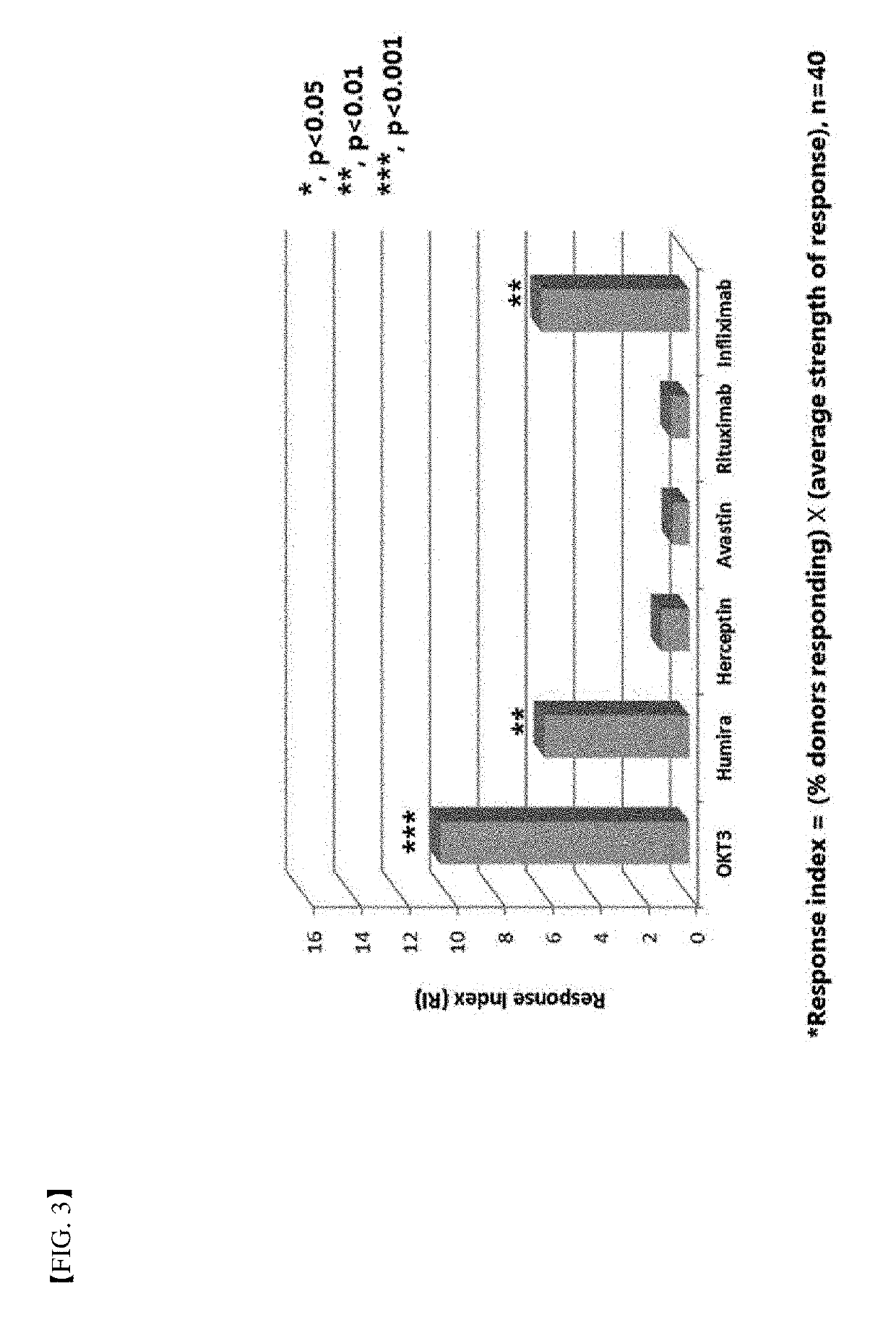
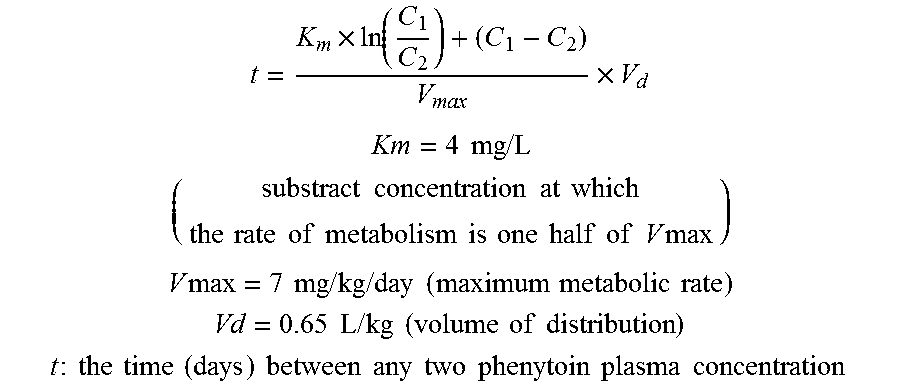Patents
Literature
30 results about "HLA-DRB1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
HLA class II histocompatibility antigen, DRB1 beta chain is a protein that in humans is encoded by the HLA-DRB1 gene. DRB1 encodes the most prevalent beta subunit of HLA-DR. Several alleles of DRB1 (shared epitope alleles) are associated with an increased incidence of rheumatoid arthritis.
Human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and HLA gene sequencing and typing method
InactiveCN101962676AWide detection coverageResolving Ambiguous Results IssuesMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsHLA-B gene
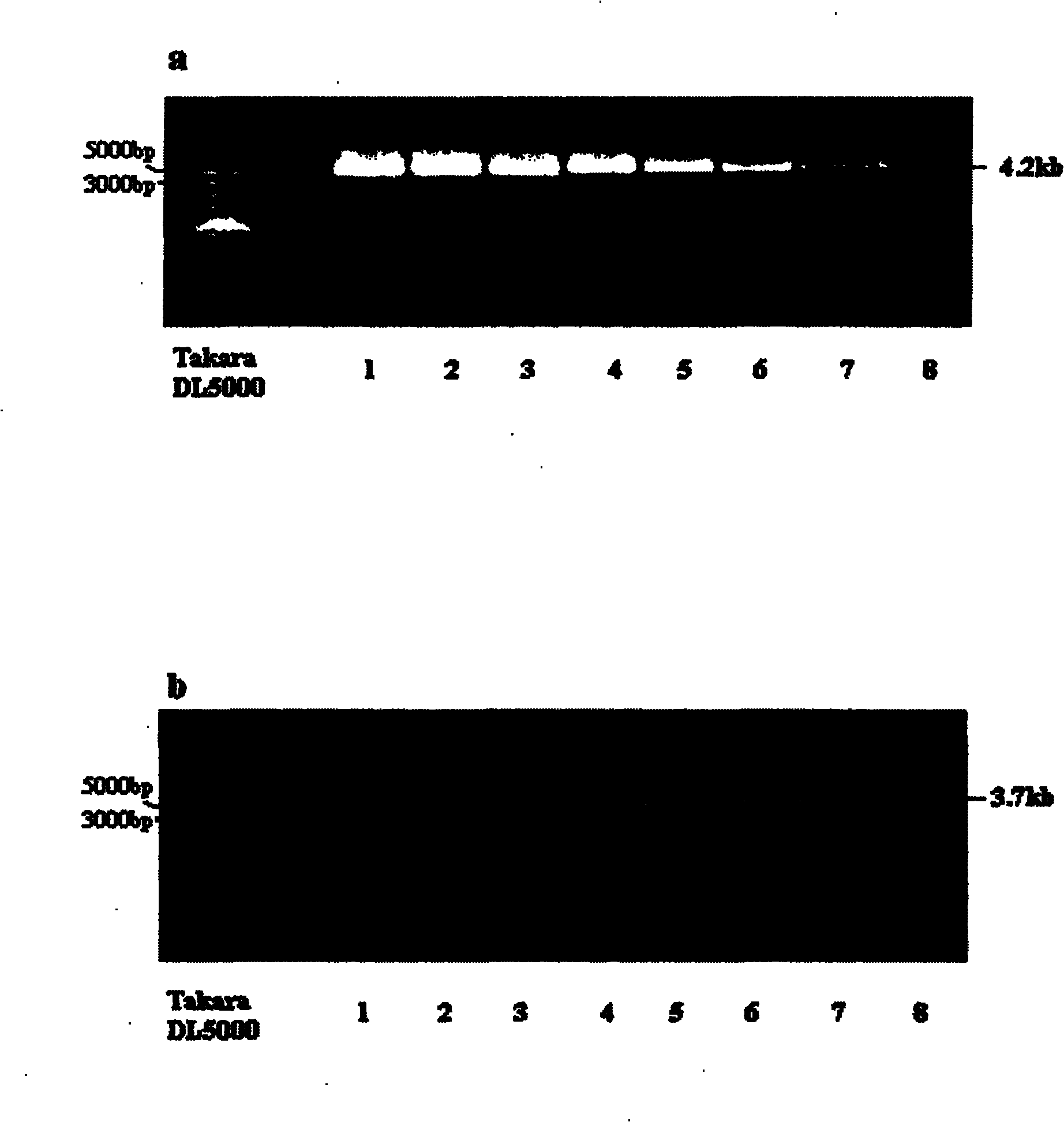
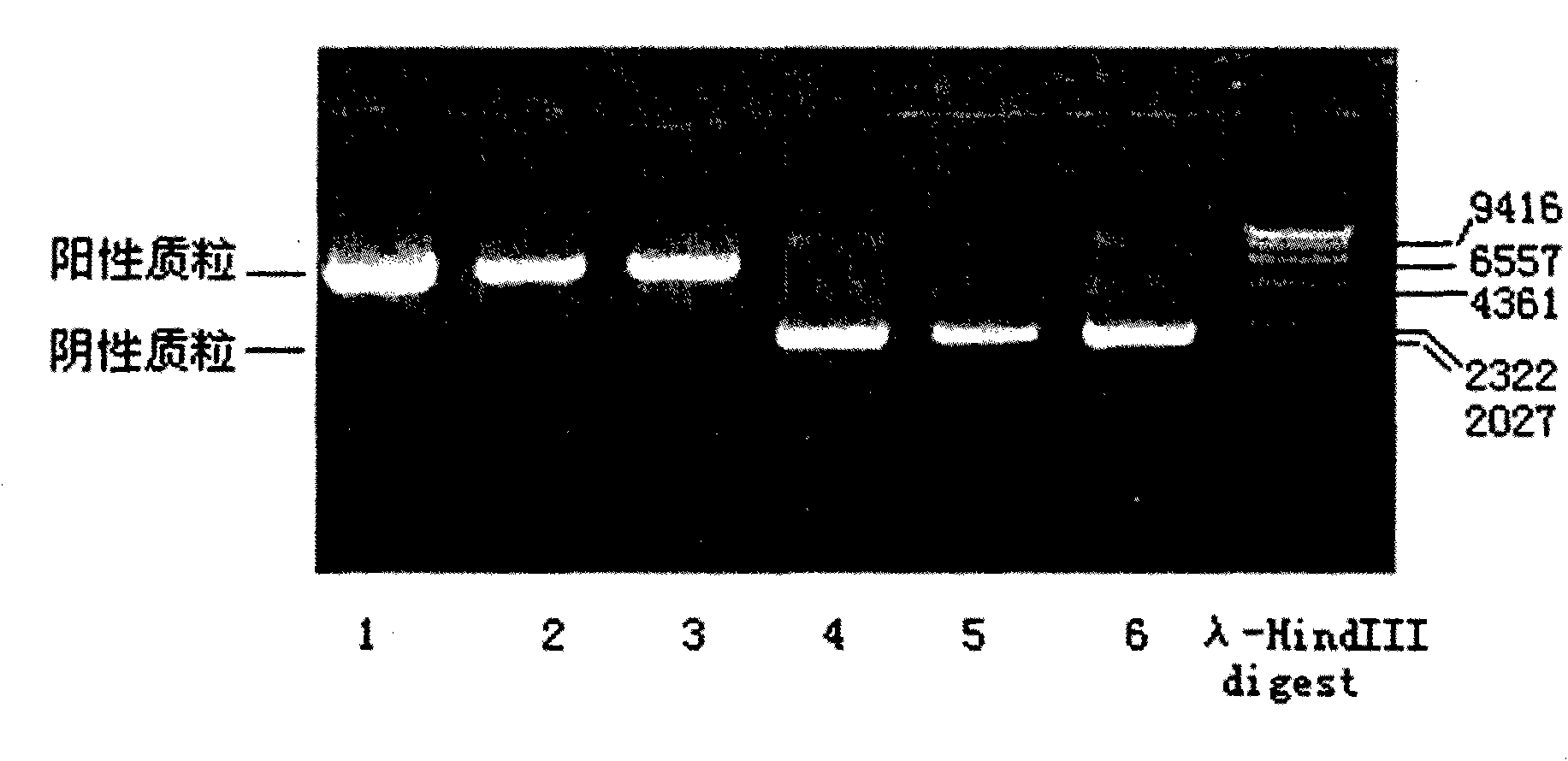
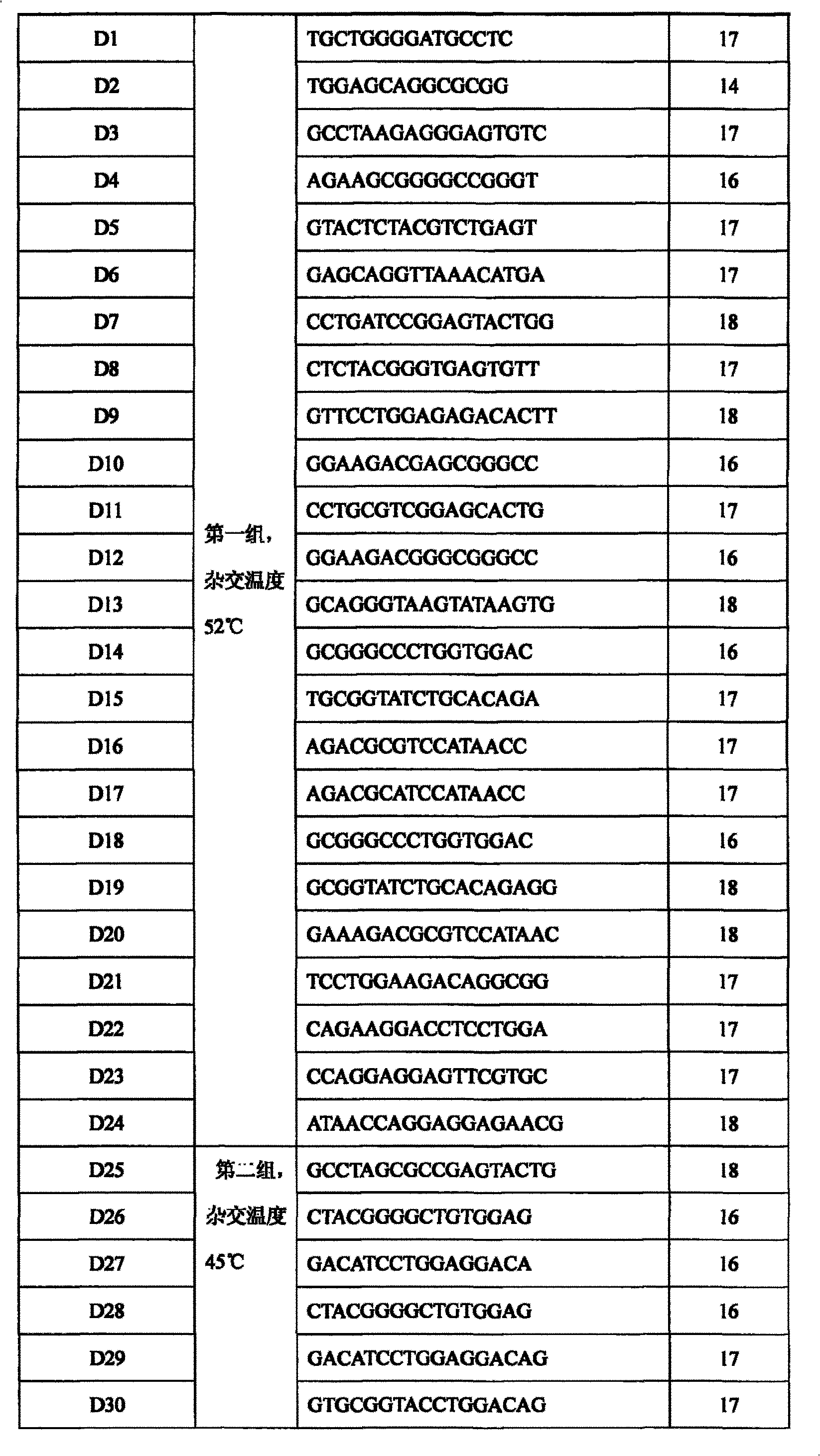
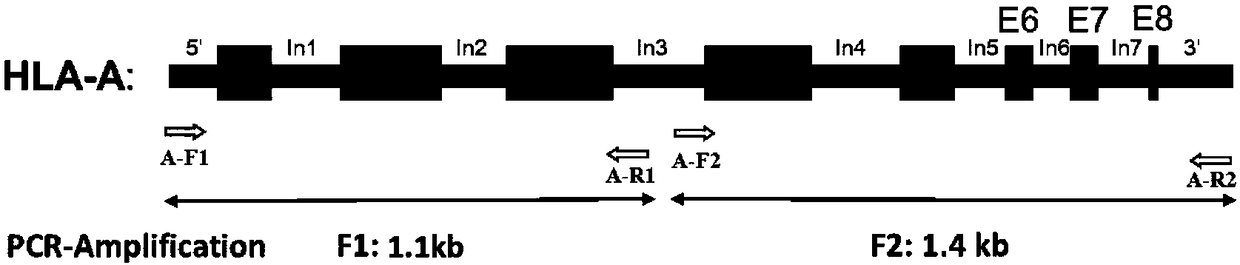
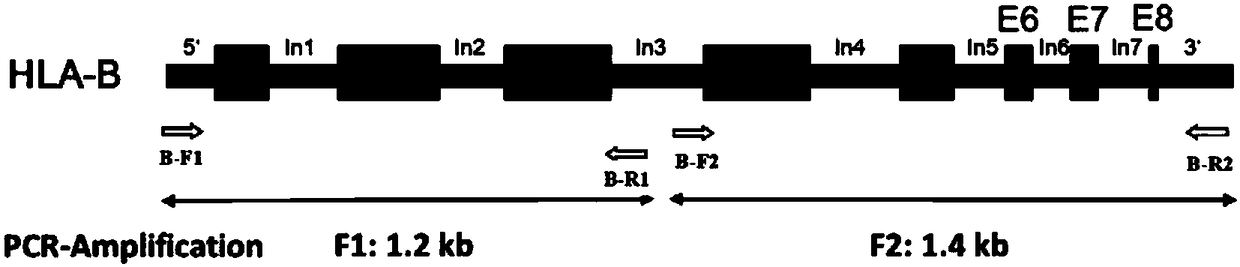
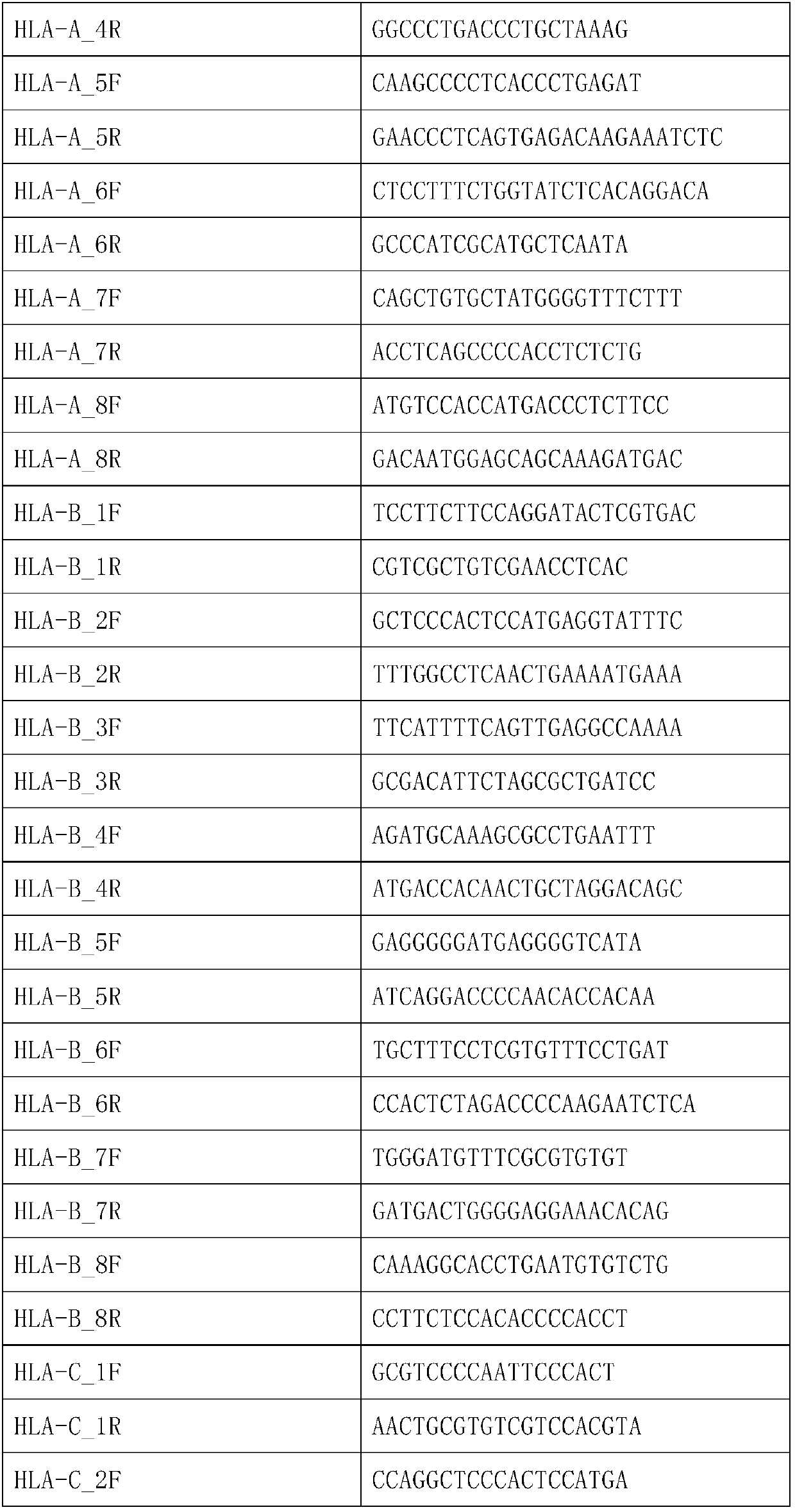
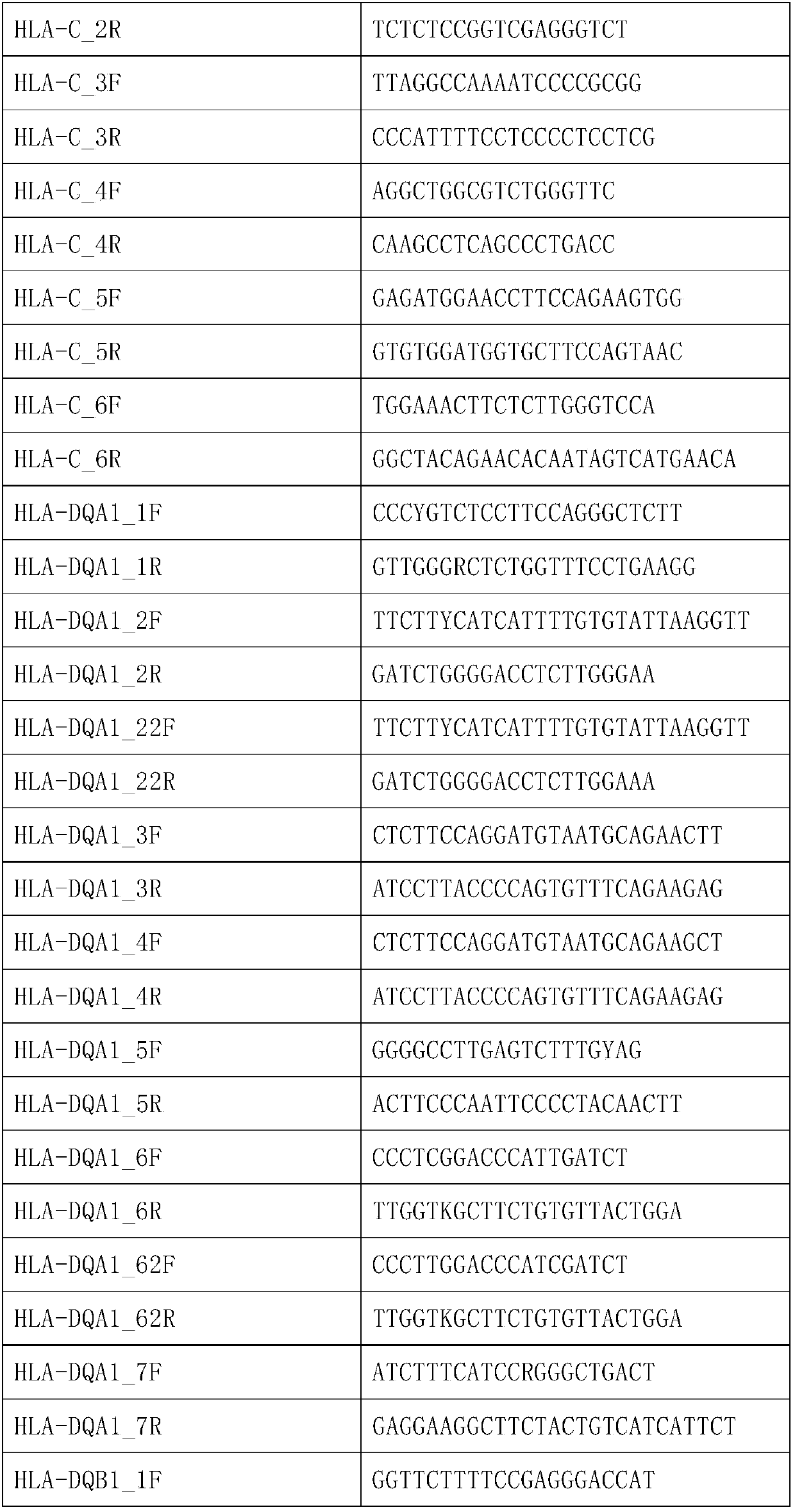
The invention discloses a human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and an HLA gene sequencing and typing method. The HLA-A and HLA-B gene full-length sequencing method comprises the following steps of: a, performing PCR amplification on about 4kb full-length sequences of HLA-A and HLA-B genes by using a pair of primers respectively; and b, cloning the amplification products to a pGEM-Tea sy vector, sequencing the full-length sequences by using ten walking sequencing primers in positive and negative directions respectively, and totally obtaining 38 allele 4.3kb full-length sequences of the HLA-A and 30 allele 3.7kb full-length sequences of the HLA-B. The HLA-A and HLA-B sequencing and typing method comprises the following steps of: performing PCR amplification on typing target areas of the HLA-A and HLA-B by using two pairs of primers respectively; and performing two directional sequencing on products by using fourteen sequencing primers respectively, wherein the HLA-DRB1 and HLA-DQB1 sequencing and typing method comprises the following steps of: amplifying sequences of second and third exons of DRB1 and DQB1 by adopting group specificity primers respectively; performing two directional sequencing on the second and third exons of the DRB1 by adopting eight group specificity primers and three sequencing primers; and performing two directional sequencing on the second and third exons of the DQB1 by adopting four sequencing primers respectively.
Owner:SHENZHEN BLOOD CENT
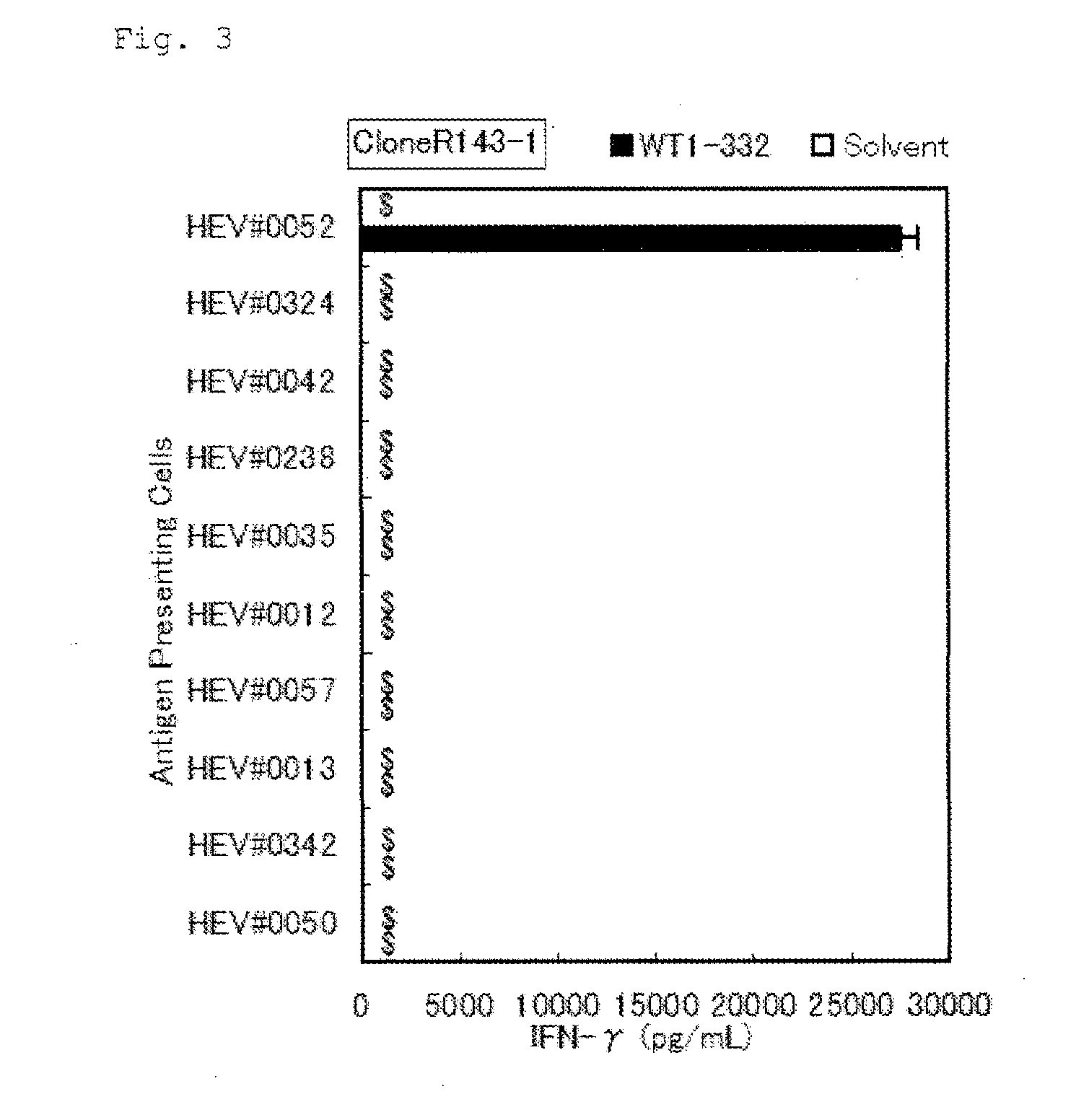
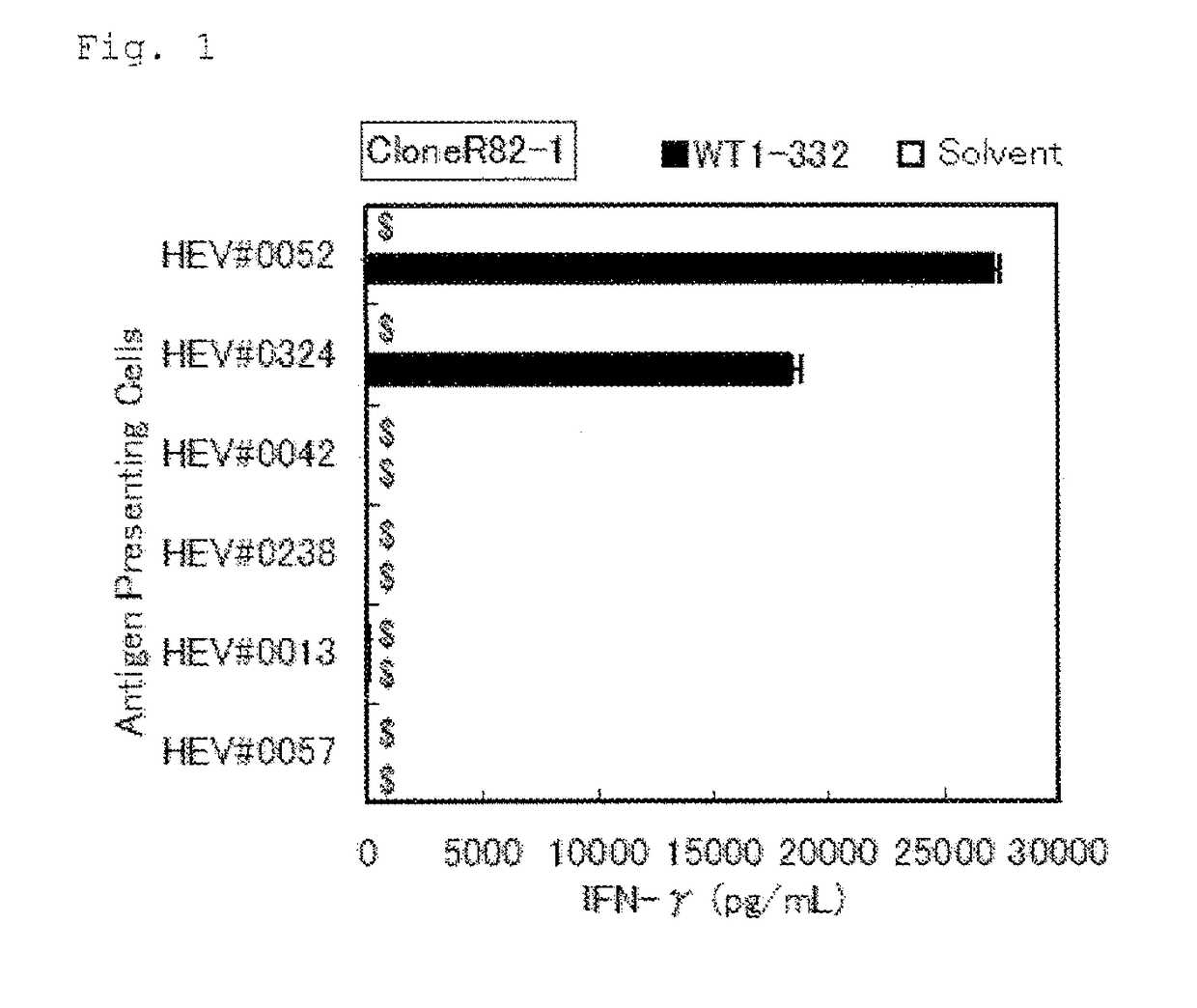
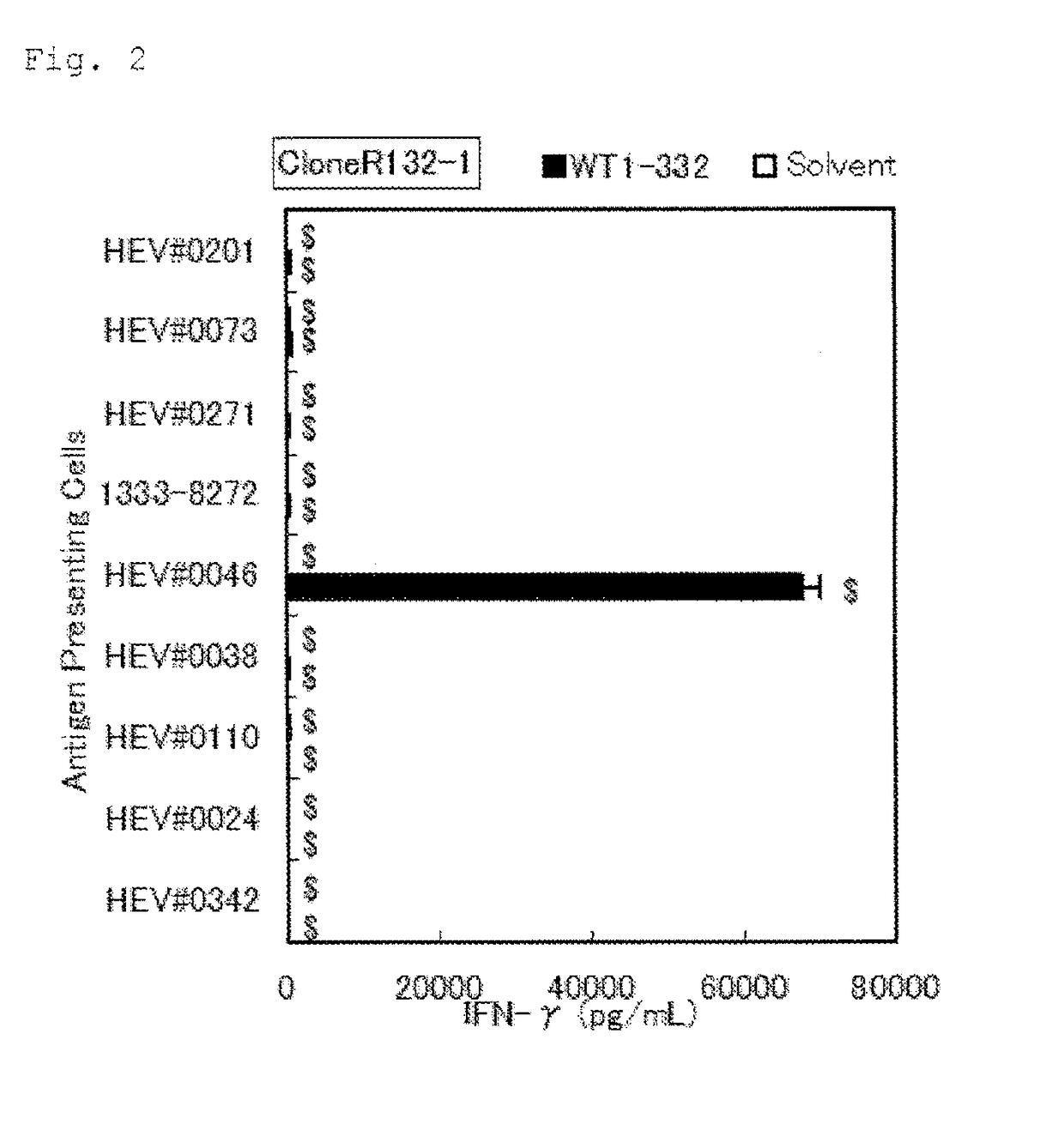
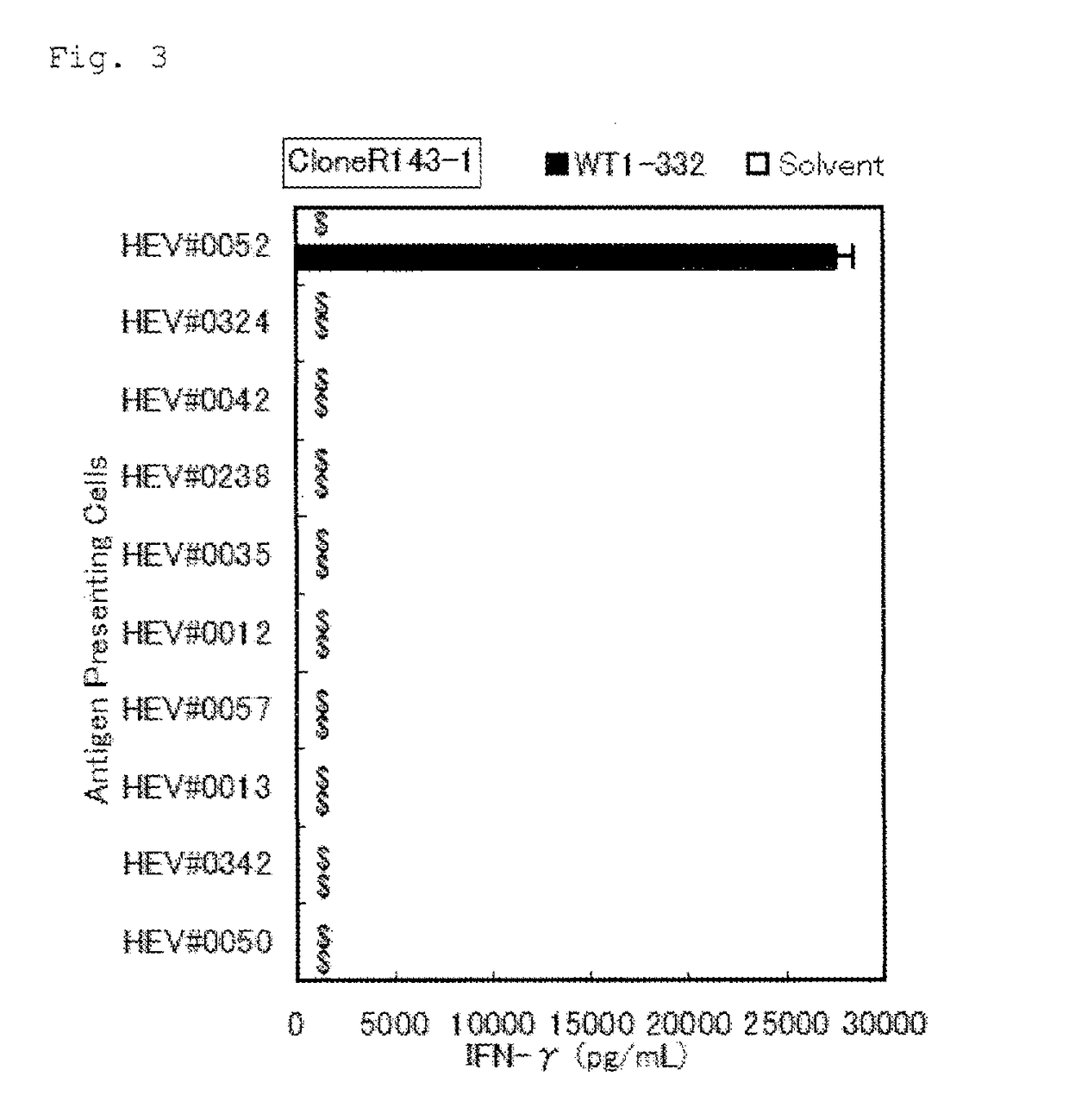
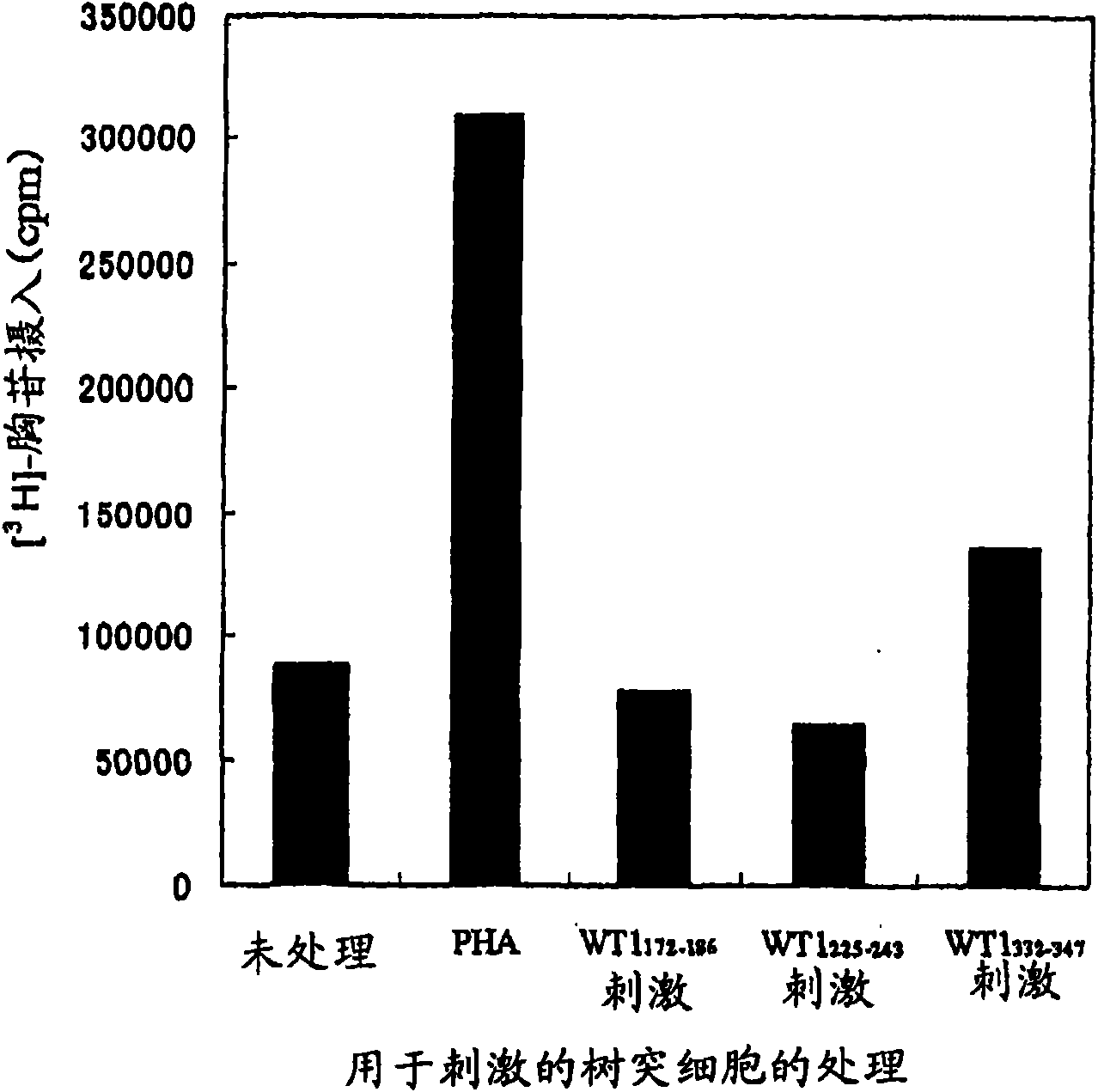
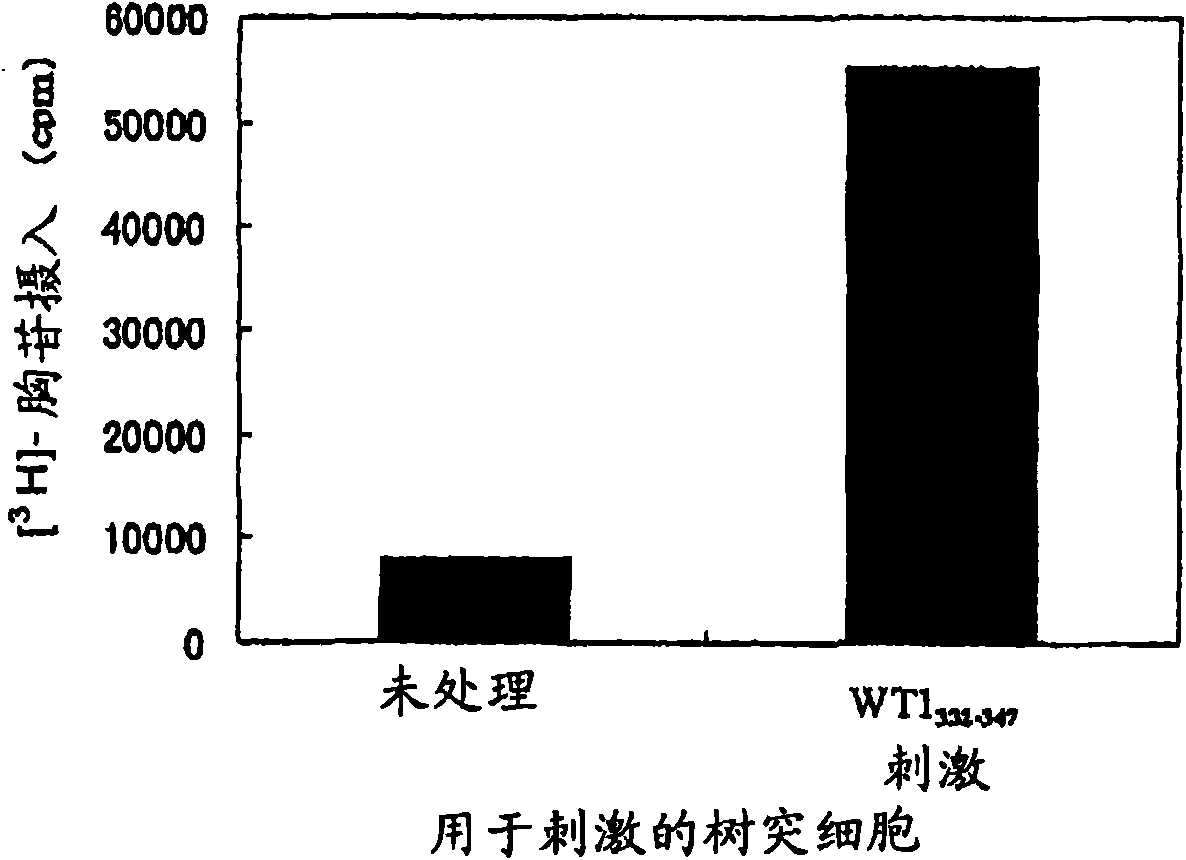
Method for activating helper t cell
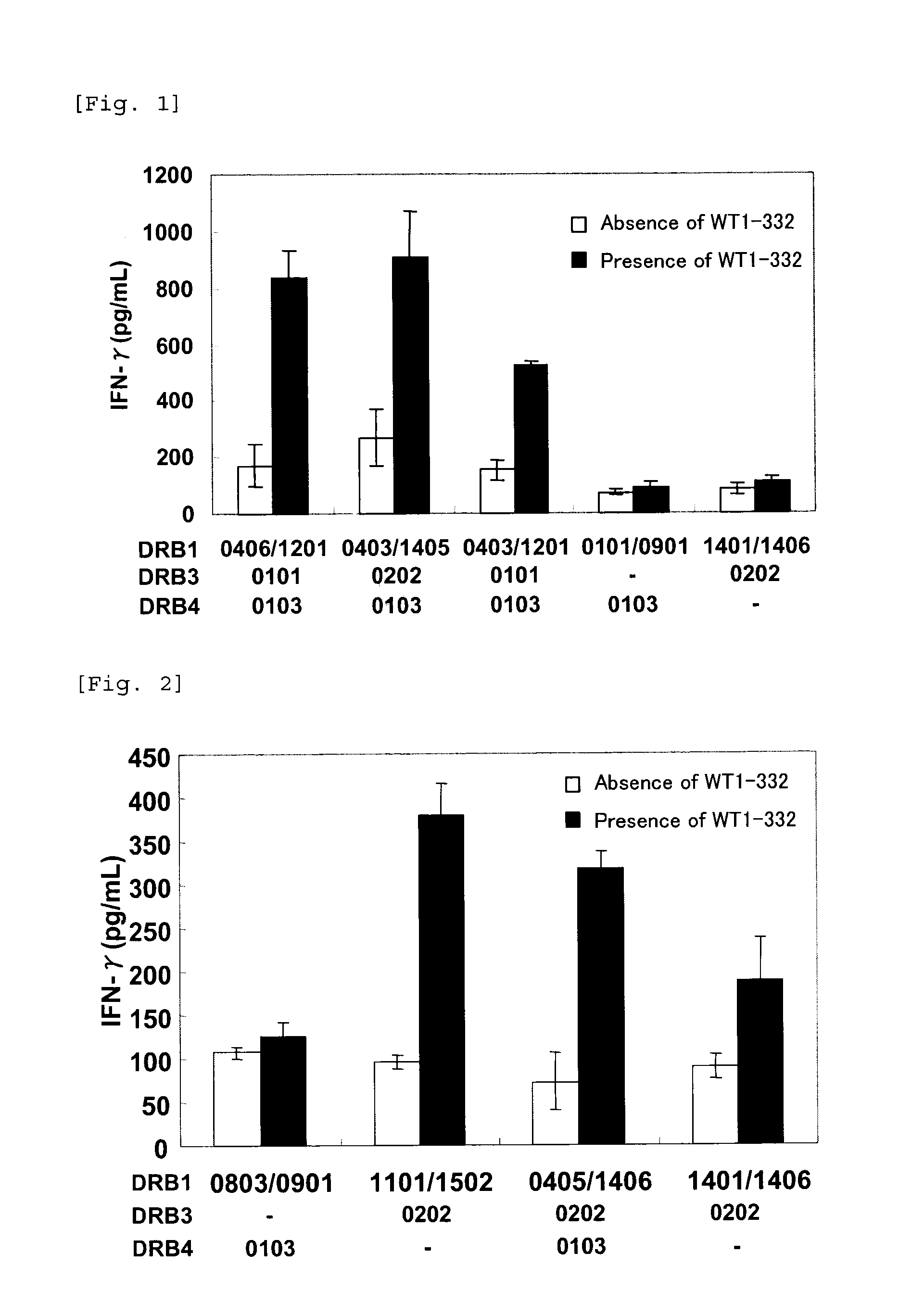
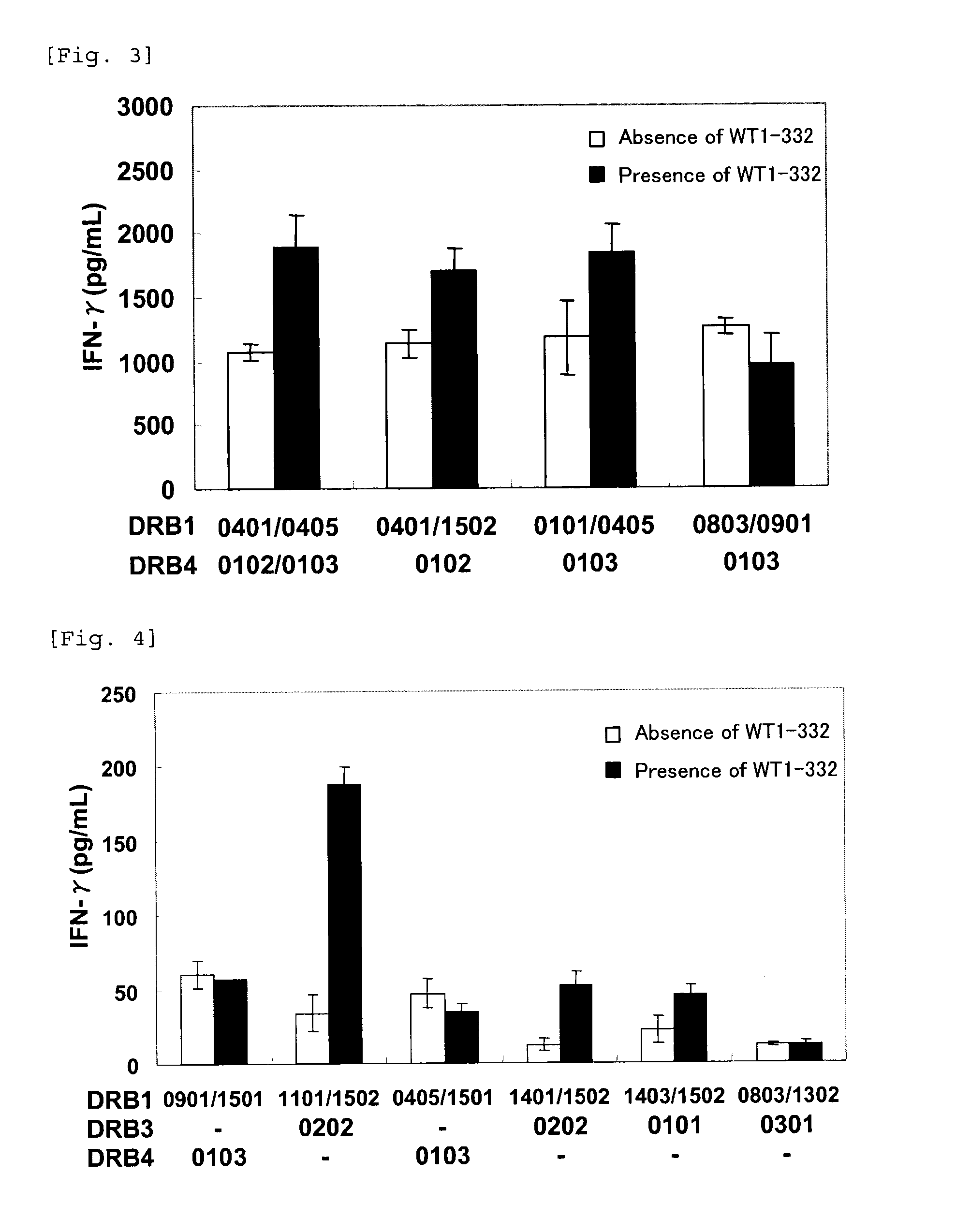
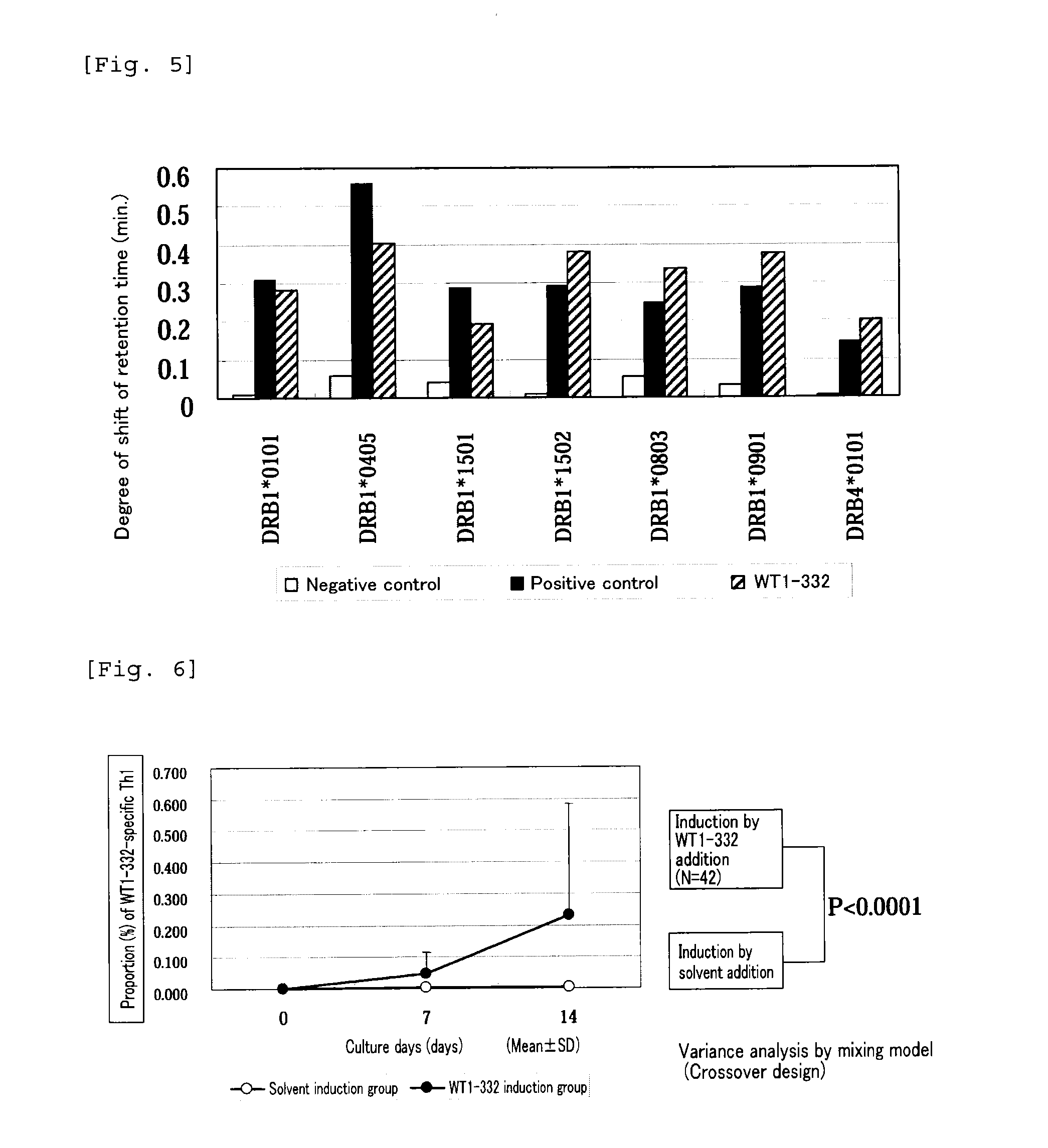
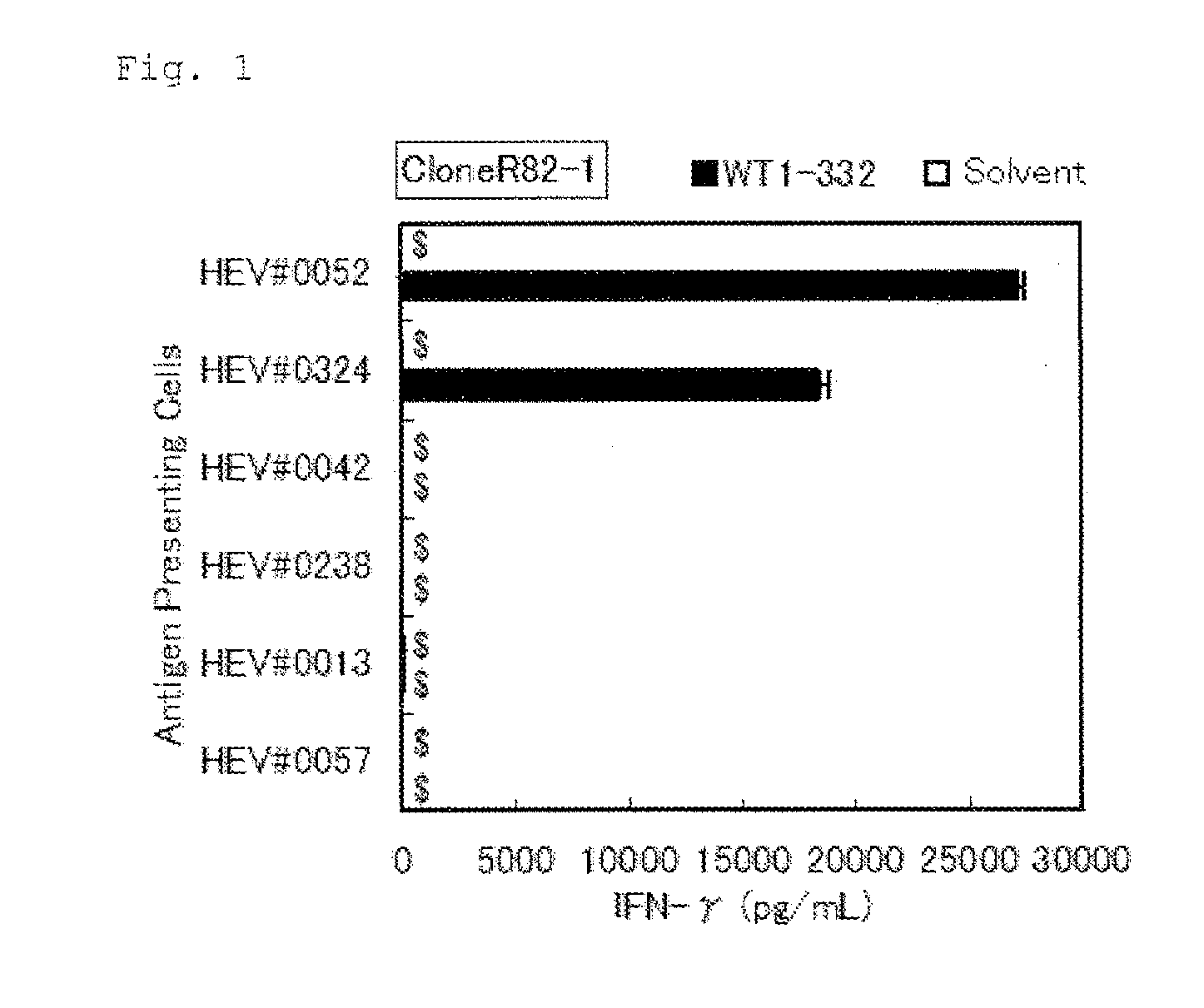
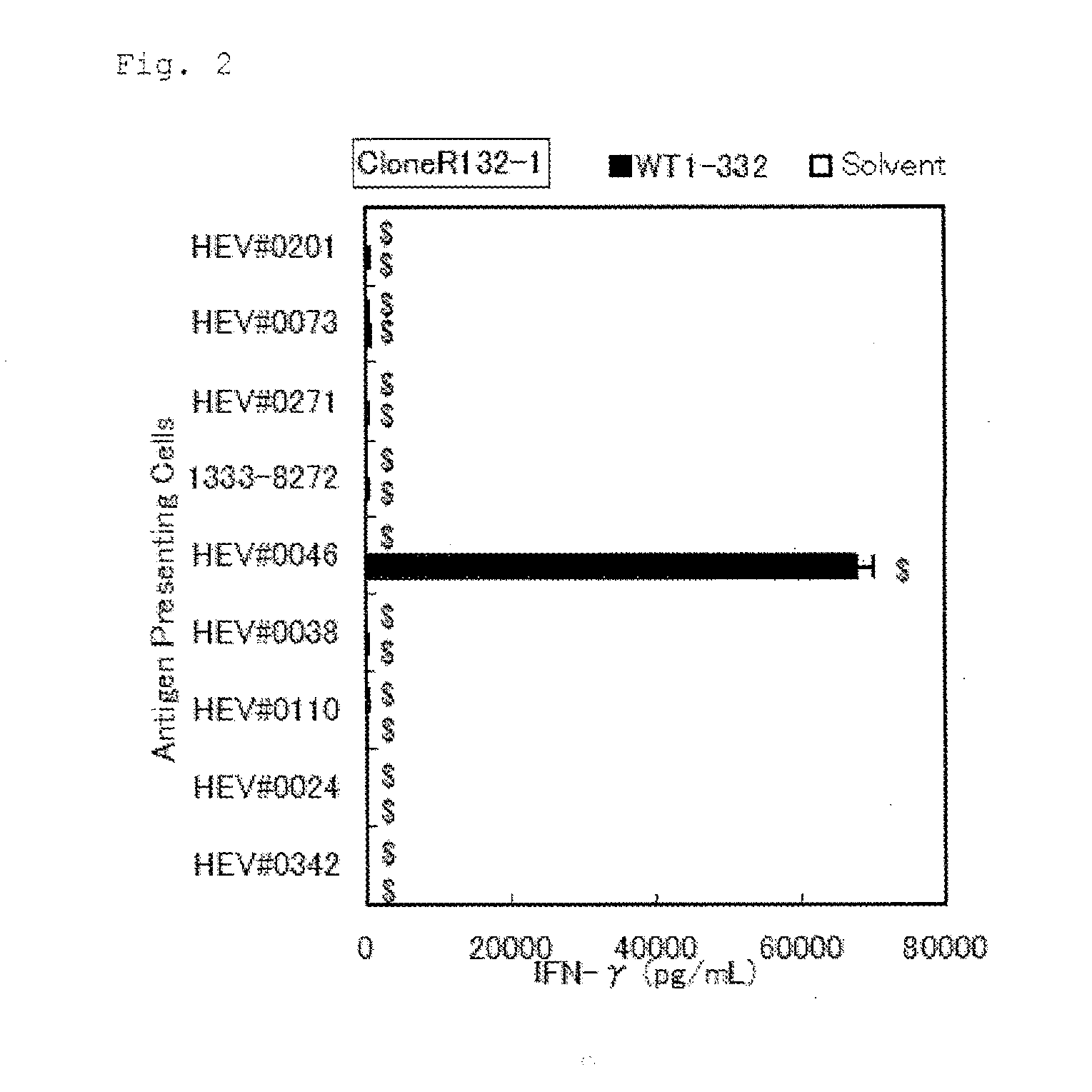
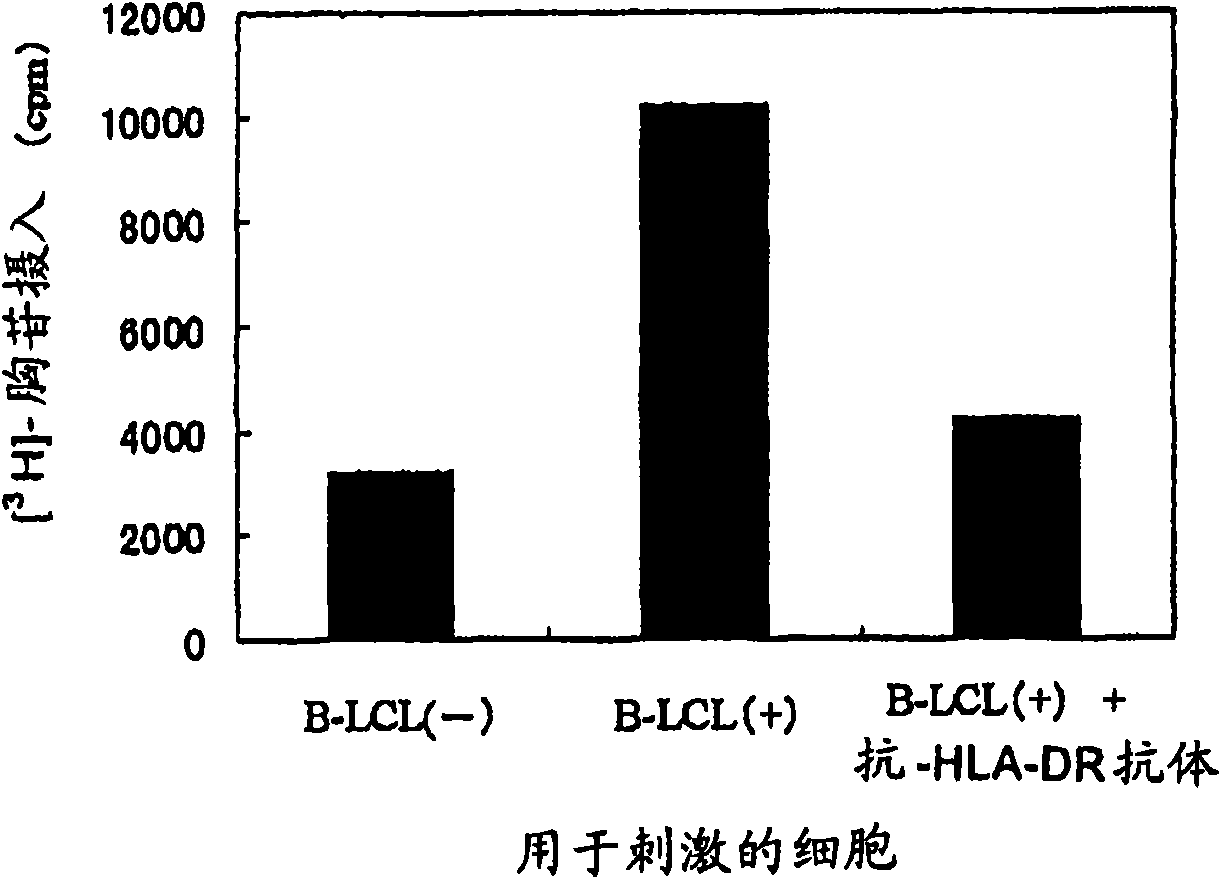
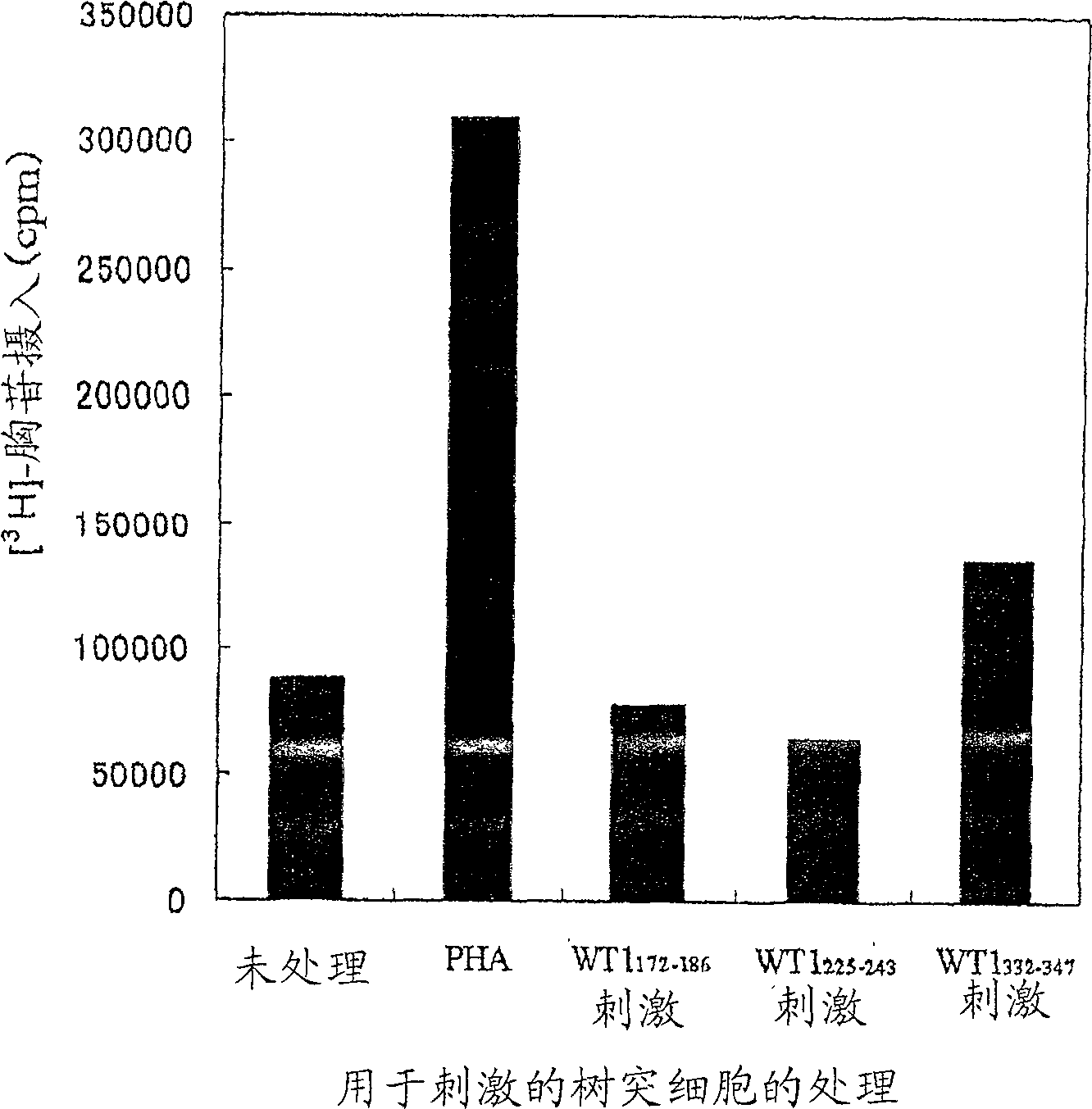
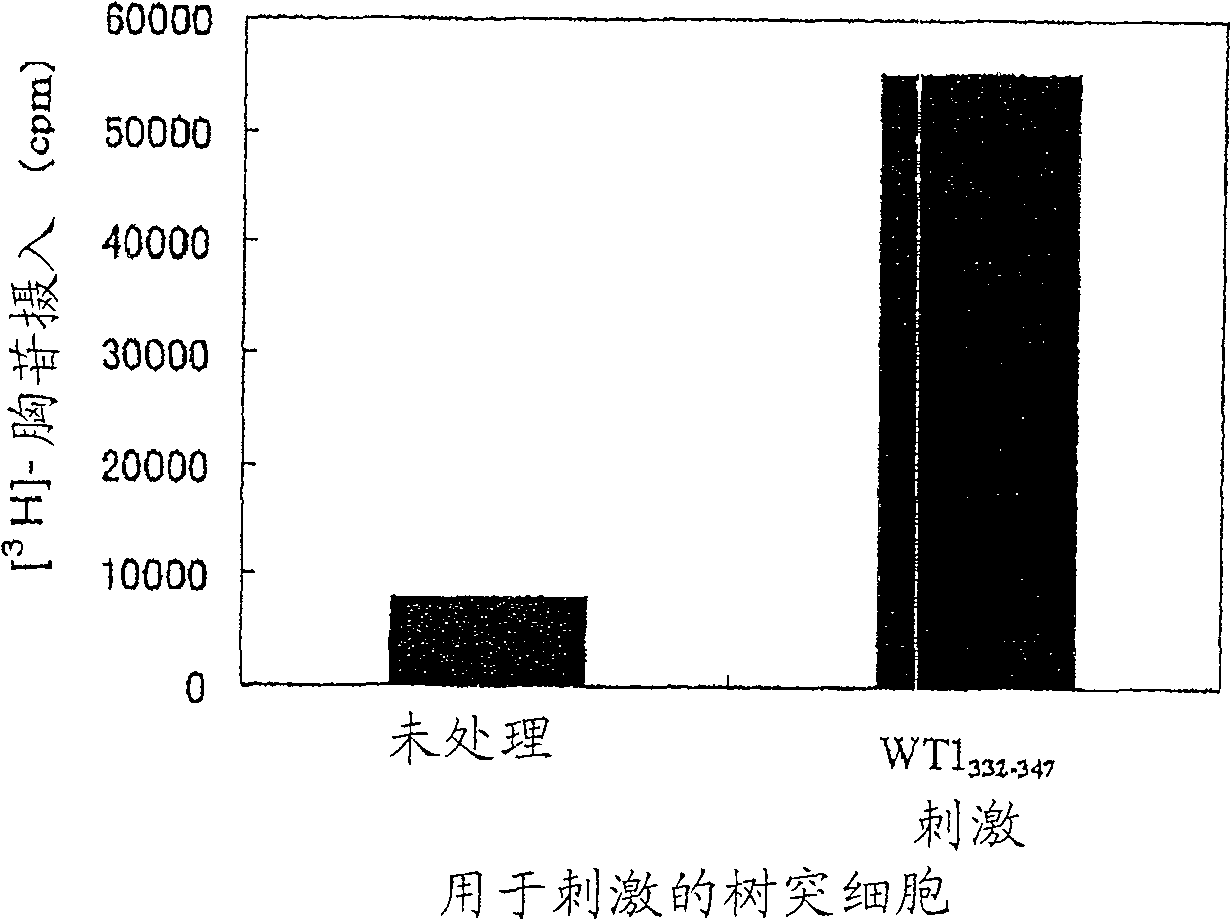
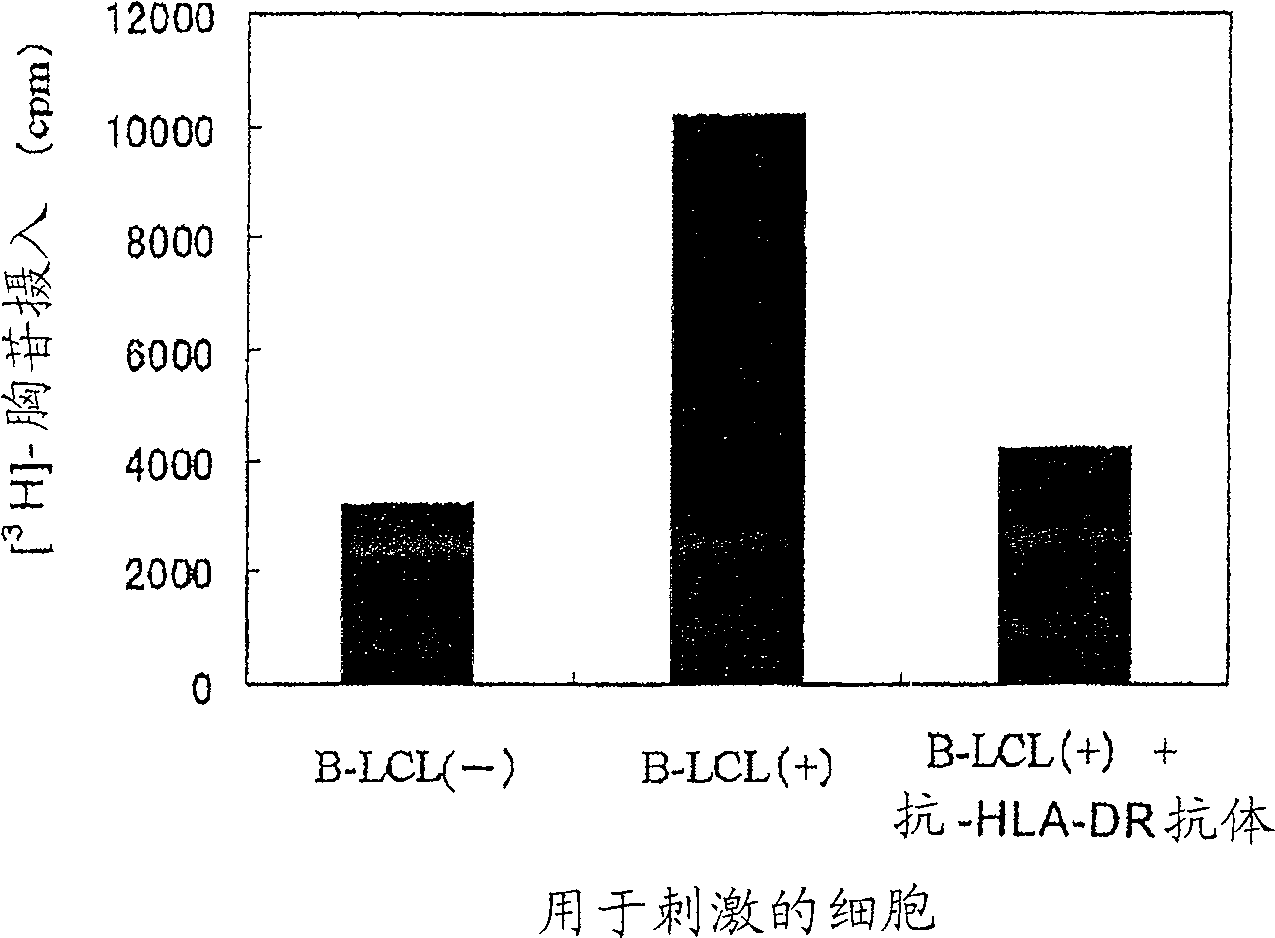
The present invention relates to a method for activating helper T cells, which includes the step of activating helper T cells by adding a WT1 peptide to antigen presenting cells, wherein the WT1 peptide has the ability to bind to any MHC class II molecule of an HLA-DRB1* 0101 molecule, an HLA-DRB1* 0401 molecule, an HLA-DRB1* 0403 molecule, an HLA-DRB1* 0406 molecule, an HLA-DRB1* 0803 molecule, an HLA-DRB1* 0901 molecule, an HLA-DRB1* 1101 molecule, an HLA-DRB3* 0202 molecule, an HLA-DRB4* 0101 molecule, an HLA-DPB1* 0201 molecule or an HLA-DPB1* 0301 molecule, and the like.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Method for activating helper t cell
The present invention relates to a method for activating helper T cells, which includes the step of activating helper T cells by adding a WT1 peptide to antigen presenting cells, wherein the WT1 peptide has the ability to bind to an MHC class II molecule selected from HLA-DRB1*08:02 molecule, an HLA-DRB1*13:02 molecule, an HLA-DRB1*14:03 molecule, an HLA-DRB1*14:05 molecule, an HLA-DQB1*03:02 molecule, and an HLA-DQB1*04:01 molecule.
Owner:INT INST OF CANCER IMMUNOLOGY INC
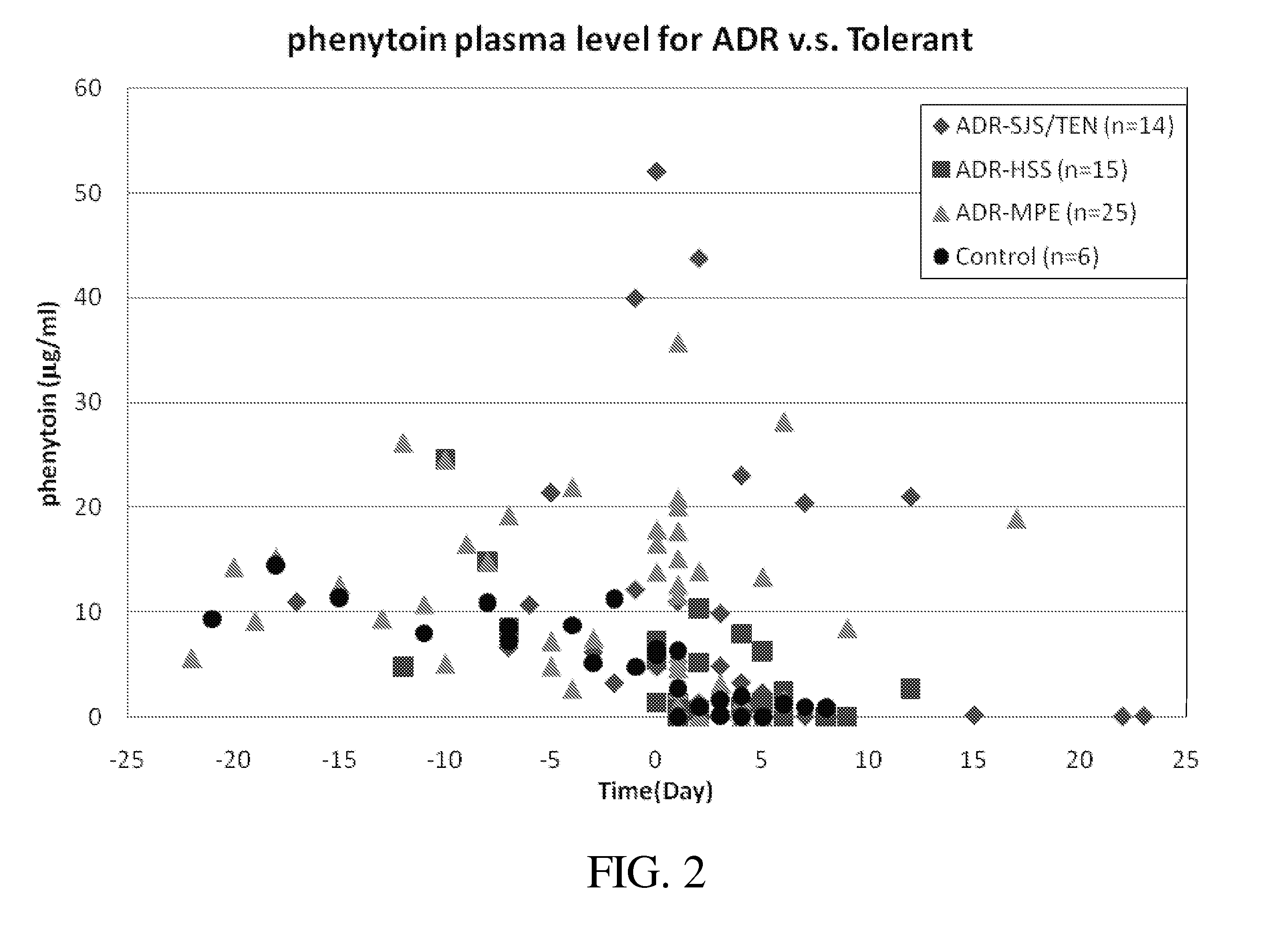
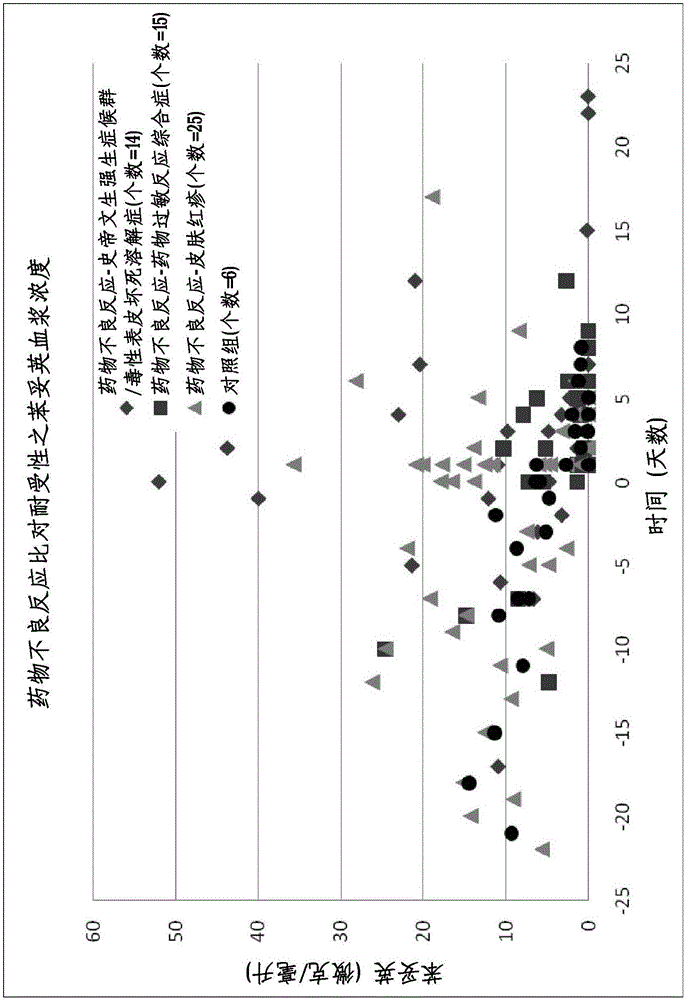
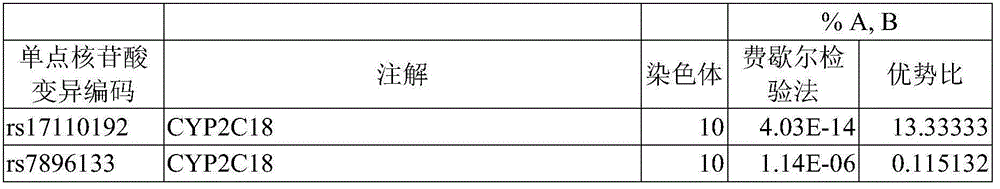
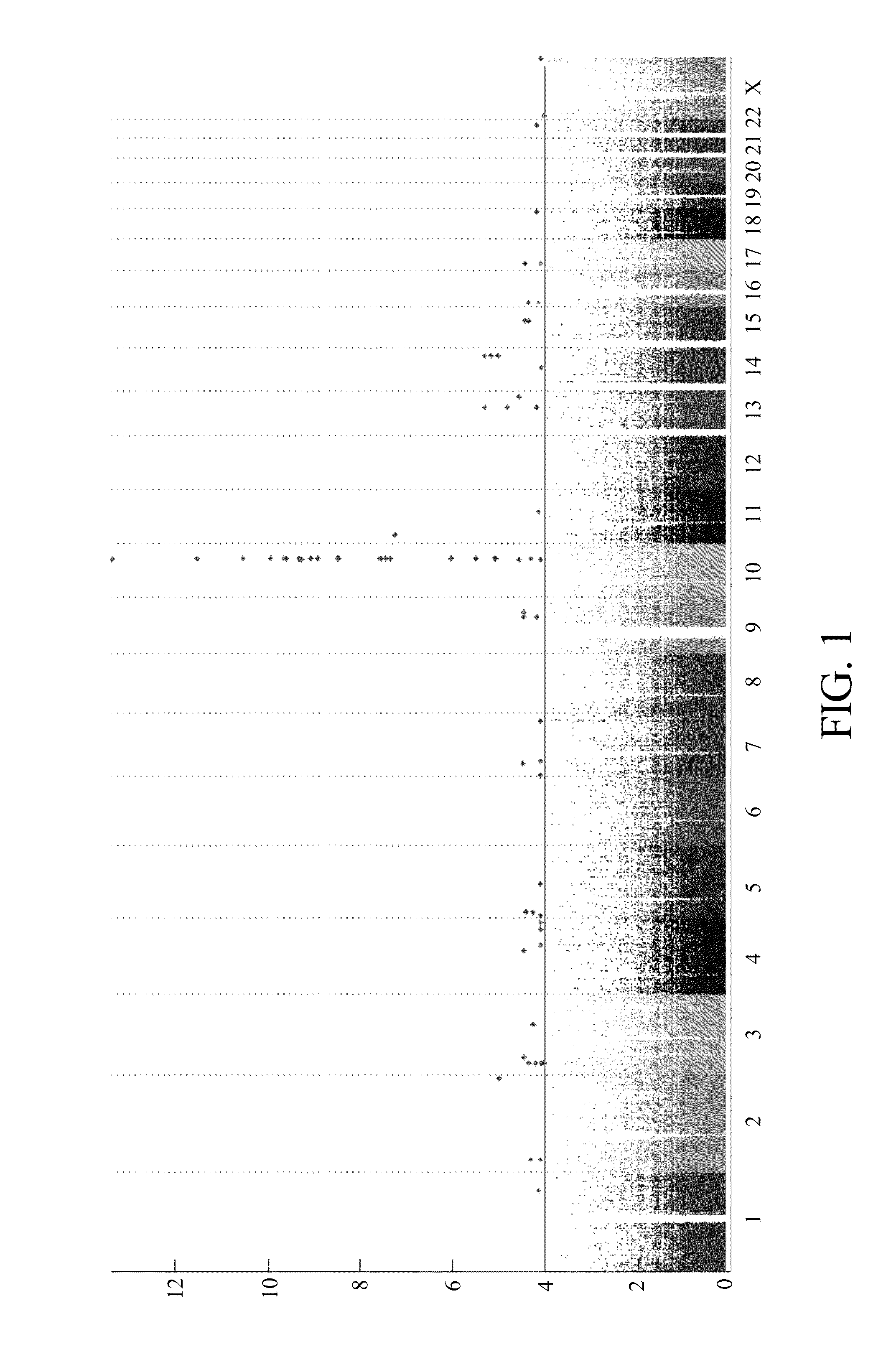
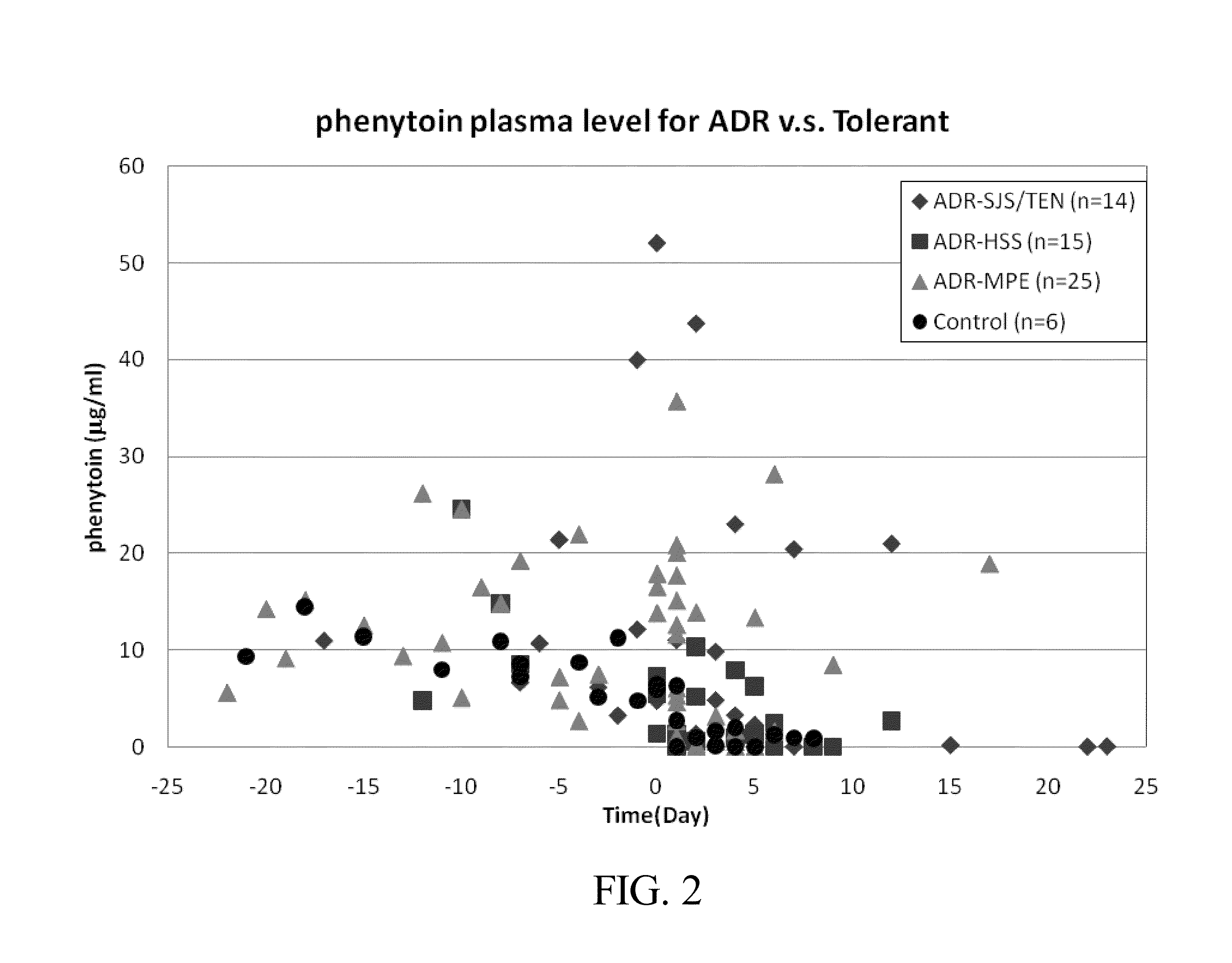
Risk assessment for phenytoin-induced adverse drug reactions
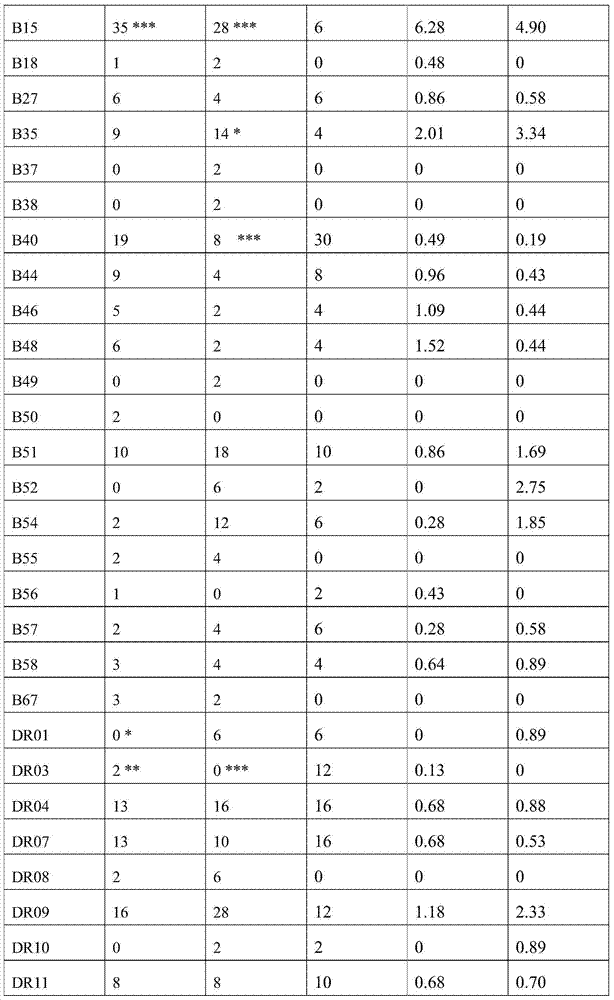
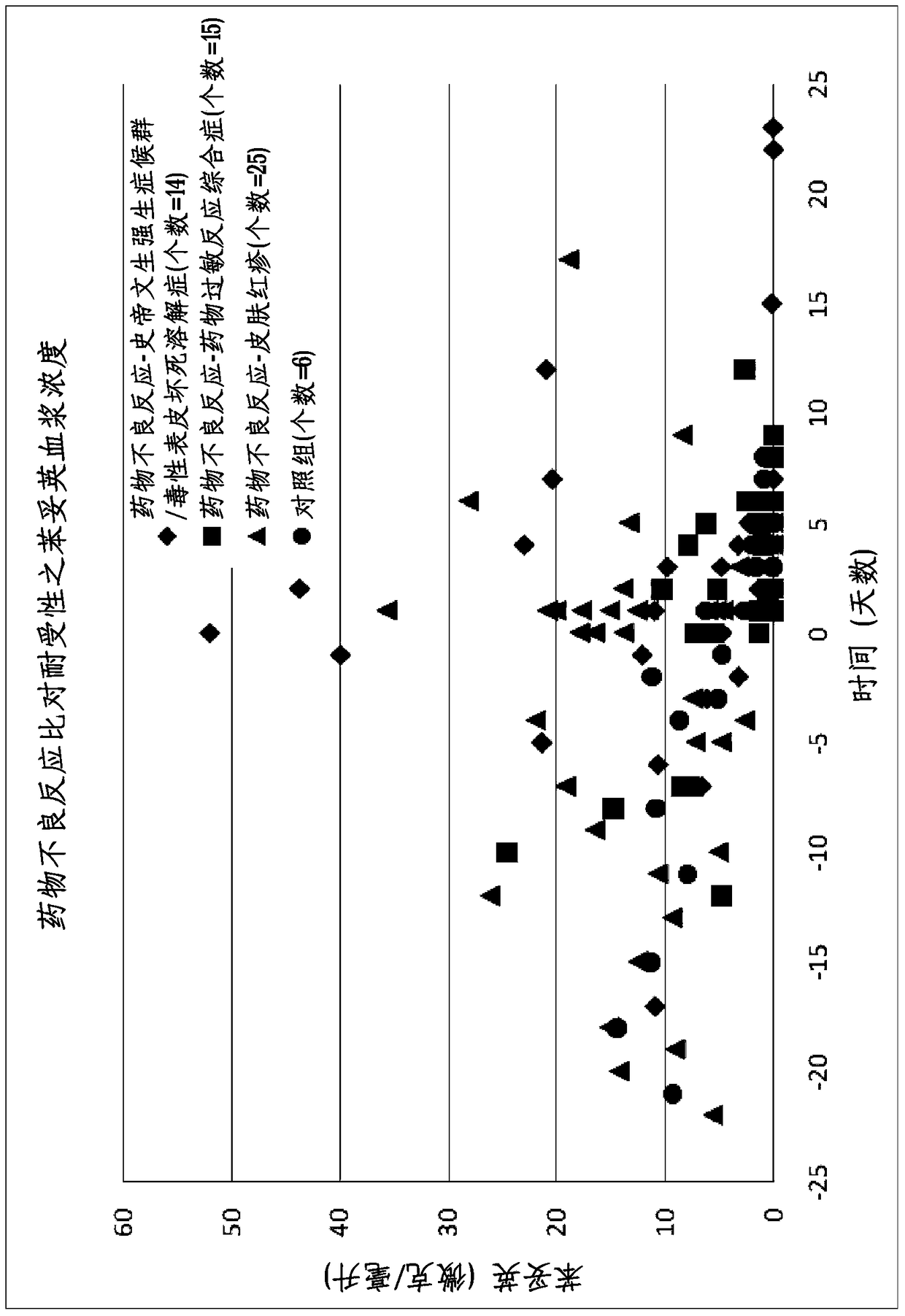
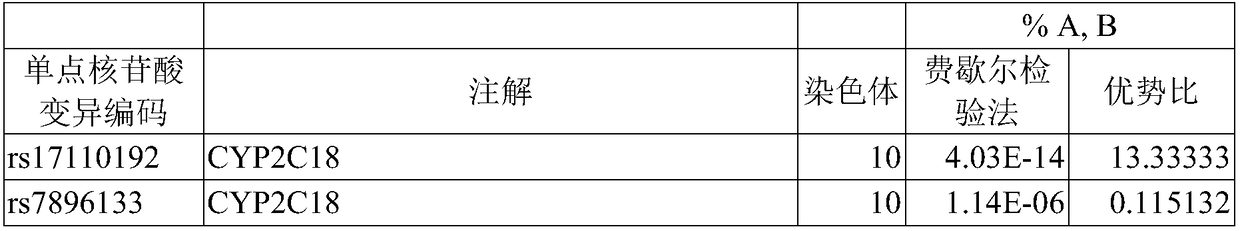
A method of predicting the risk of a patient for developing phenytoin-induced adverse drug reactions (ADRs), including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), or drug reactions with eosinophilia and systemic symptoms (DRESS) is disclosed. Genetic polymorphisms of CYP2C genes (including CYP2C9, CYP2C19, CYP2C8 and CYP2C18), HLA alleles (including HLA-A*0207, HLA-A*2402, HLA-B*1301, HLA-B*1502, HLA-B*4001, HLA-B*4609, HLA-B*5101, HLA-DRB1*1001 or HLA-DRB1*1502) and phenytoin concentration in the patient's plasma can all contribute to phenytoin-induced ADRs.
Owner:CHANG GUNG MEDICAL FOUND CHANG GUNG MEMORIAL HOSPITAL AT KEELUNG
Methods of predicting thiopurine response
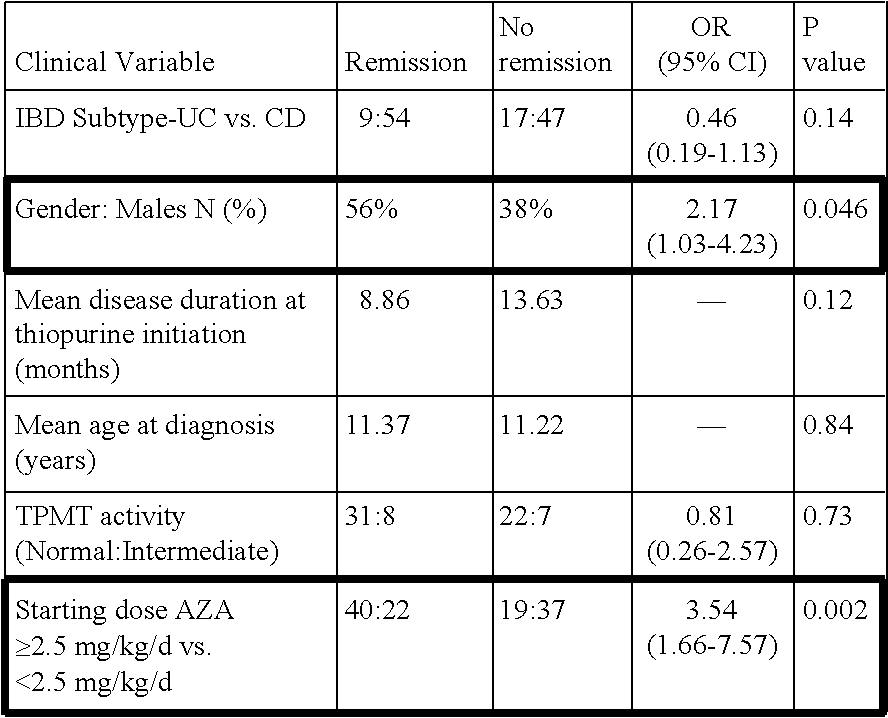
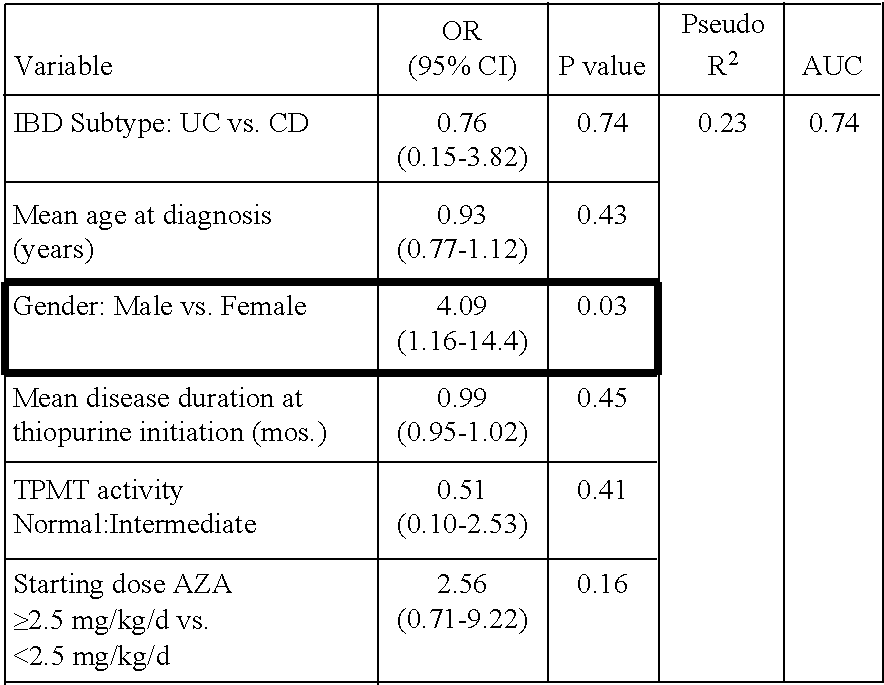
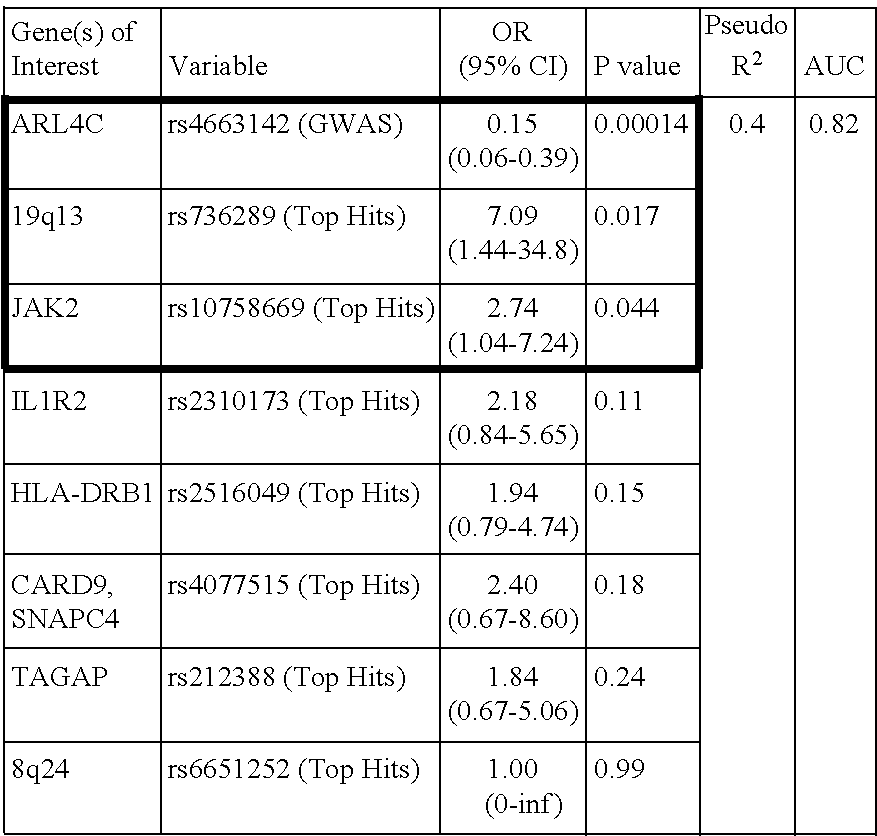
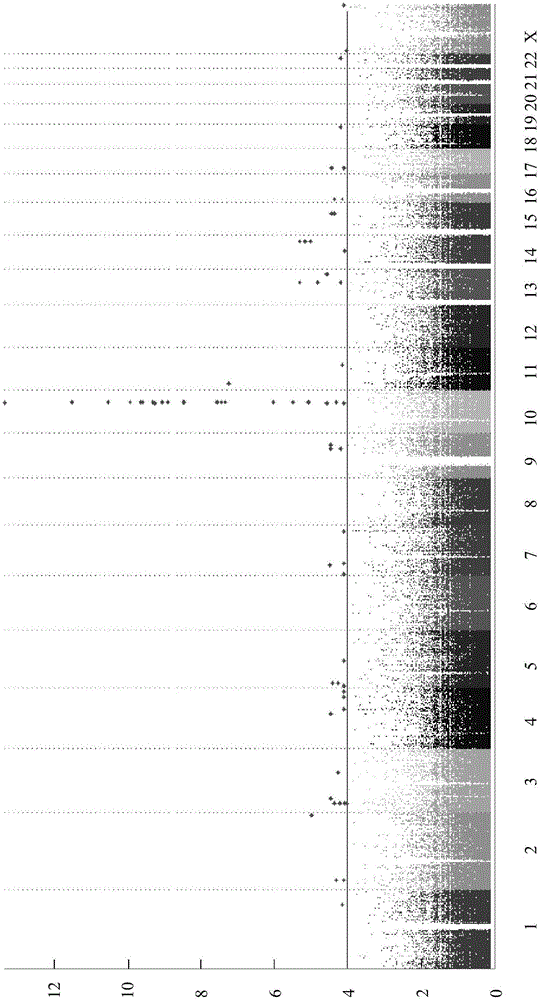
The present invention relates to methods of predicting therapeutic efficacy of thiopurines in an individual by determining the presence of one or more risk variants. In one embodiment, the effective therapeutic efficacy of thiopurines is determined by the presence of risk variants at the genetic loci of HLA-DRB1, CREM, TAGAP, PLCL1, GPX4, SBNO2, MEF2A and / or LYSMD4. In another embodiment, the risk variants are located at the genetic loci of ARL4C, IL1R2, JAK2, 19q13, CARD9, SNAPC4, and / or 8q24. In another embodiment, the individual is has been diagnosed with inflammatory bowel disease.
Owner:CEDARS SINAI MEDICAL CENT
Method for evaluating drug anaphylactic reaction caused by antiepileptic drug phenytoin with HLA allele
Provided is a method for evaluating a drug anaphylactic reaction caused by antiepileptic drug phenytoin with HLA allele. The drug anaphylactic reaction comprises a Steven Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) or a drug reaction with eosinophiliaand systemic symptoms (DRESS). The gene polymorphism (including CYP2C9, CYP2C19, CYP2C8 and CYP2C18) of CYP2C genes, HLA gene types (including HLA-A*0207, HLA-A*2402, HLA-B*1301, HLA-B*1502, HLA-B*4001, HLA-B*4609, HLA-B*5101, HLA-DRB1*1001 and HLA-DRB1*1502) and the concentration of phenytoin in plasma of a patient all may cause phenytoin adverse drug reactions.
Owner:CHANG GUNG MEDICAL FOUND CHANG GUNG MEMORIAL HOSPITAL AT KEELUNG
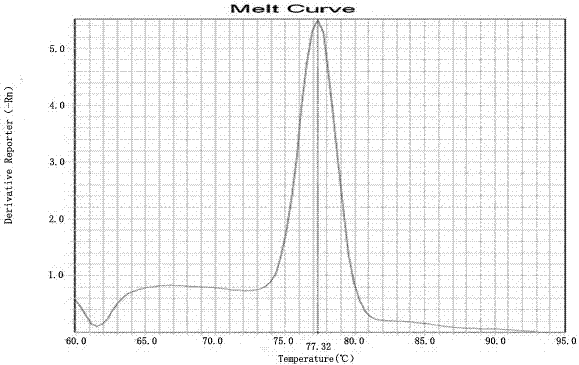
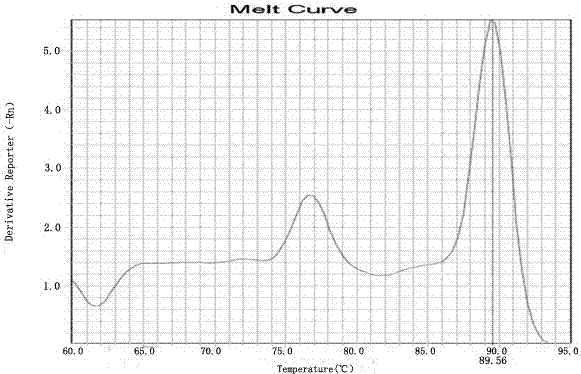
Kit for detecting HLA genotypes through fluorescent PCR melting curve assay
PendingCN107190088ANo follow-up experiments requiredQuick Results ReferenceMicrobiological testing/measurementOrgan transplantationHLA-B
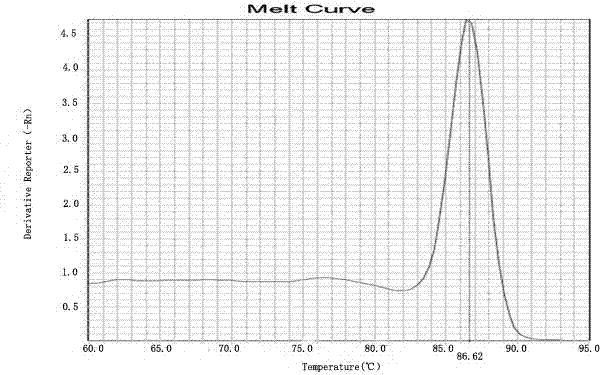
The invention provides a kit for detecting HLA genotypes through fluorescent PCR melting curve assay. The kit includes HLA-A, HLA-B, HLA-DRB1 and HLA-DQB1 genotypes, amplification is performed by using a 96-pore optical reaction plate containing primers, an amplified dual-strand DNA product is actively bonded through SYBR Green I, the low-resolution genotypes of HLA-A, HLA-B, HLA-DRB1 and HLA-DQB1 are comprehensively judged according to the result of a 96-pore fusion curve, and accordingly diagnosis of the matched types for organ transplantation is assisted.
Owner:DEBIQI BIOTECH XIAMEN
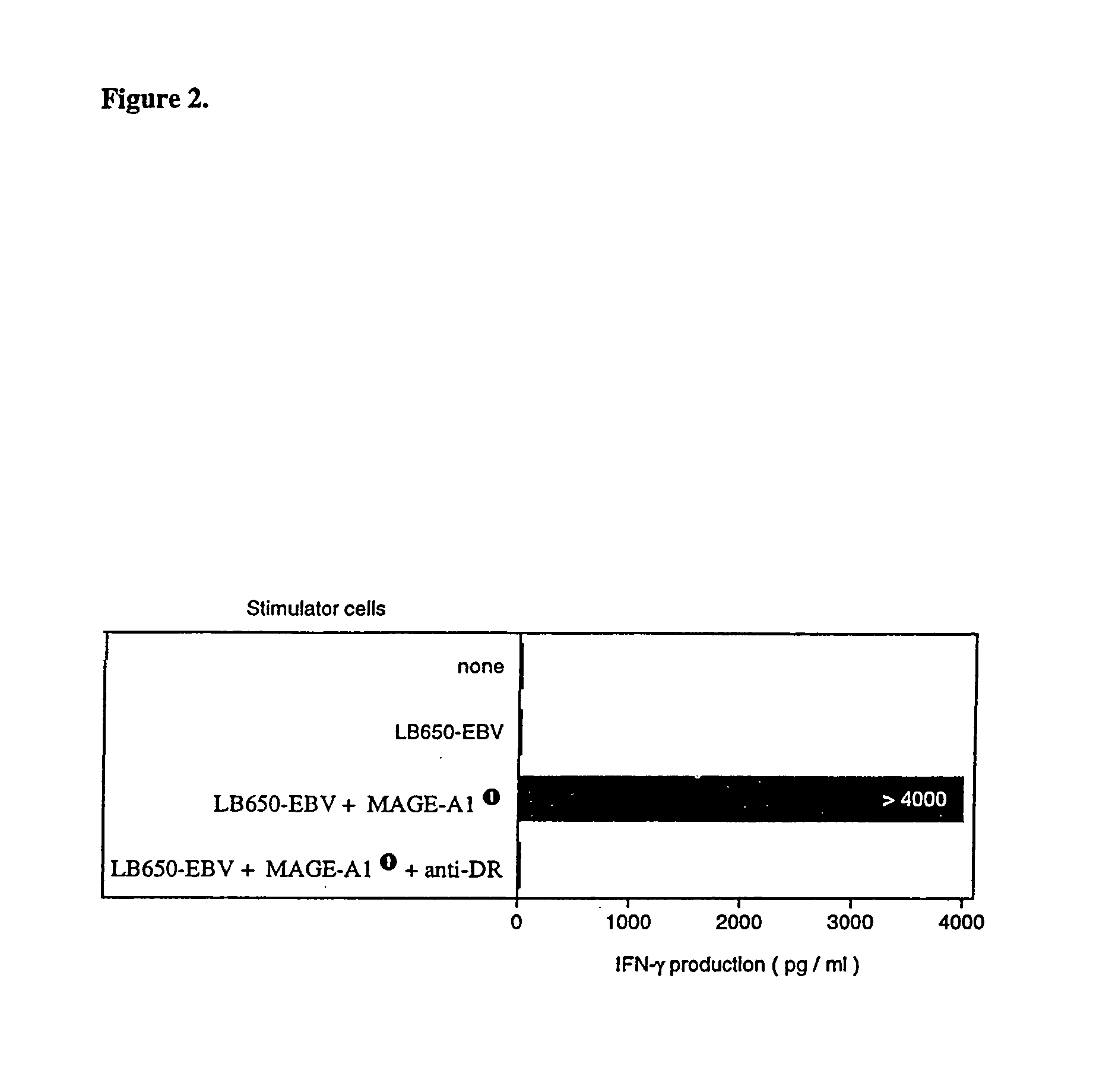
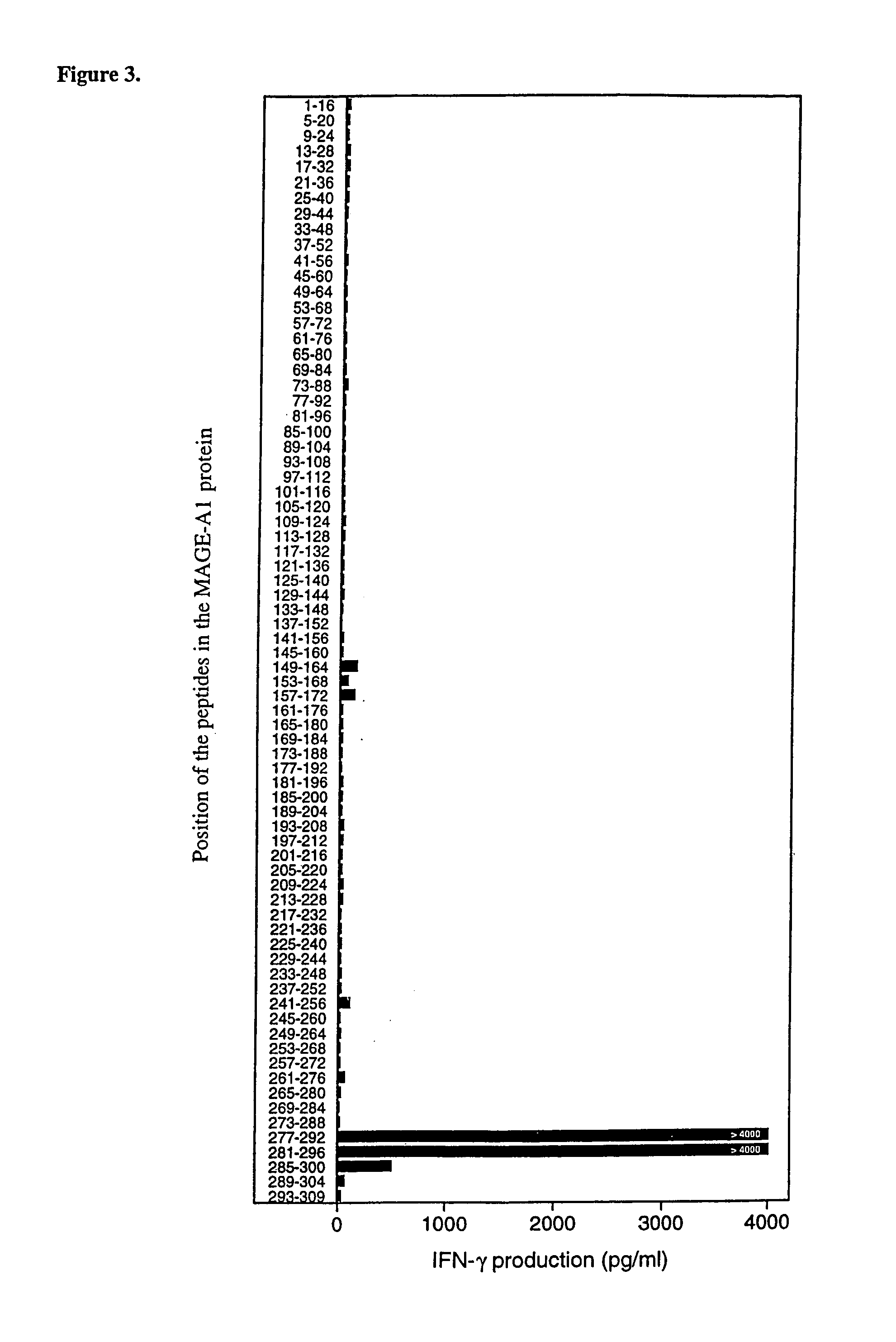
MAGE-A1 peptides presented by HLA class II molecules
InactiveUS7157091B1Induce activationInduced proliferationTumor rejection antigen precursorsPeptide/protein ingredientsHla class iiTarget signal
The invention provides isolated HLA DRB1*15-binding peptides consisting of the amino acid sequence set forth as SEQ ID NO:7 with 0–10 amino acids added to either or both ends of the amino acid sequence set forth as SEQ ID NO:7, and an endosomal targeting signal comprising an endosomal targeting portion of human invariant chain Ii or LAMP-1.
Owner:LUDWIG INST FOR CANCER RES
Method for activation of helper t cell and composition for use in the method
InactiveCN101622344ATumor rejection antigen precursorsPeptide/protein ingredientsAntigenBiological activation
Disclosed are: a method for activating a helper T cell, which comprises the step of adding a WT1 peptide to an antigen-presenting cell to activate the helper T cell, wherein the WT1 peptide is capable of binding to any one selected from an HLA-DRB1<*>1501 molecule, an HLA-DPB1<*>0901 molecule and an HLA-DPB1<*>0501 molecule; a composition for use in the method; a therapeutic and / or prophylactic method for cancer by activating a helper T cell; a pharmaceutical composition for use in the therapeutic and / or prophylactic method; and others.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Reagent kit for parting detection of HLA-DRB1 gene
ActiveCN101314790AHigh detection throughputStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationDNA Microarray ChipAntigen
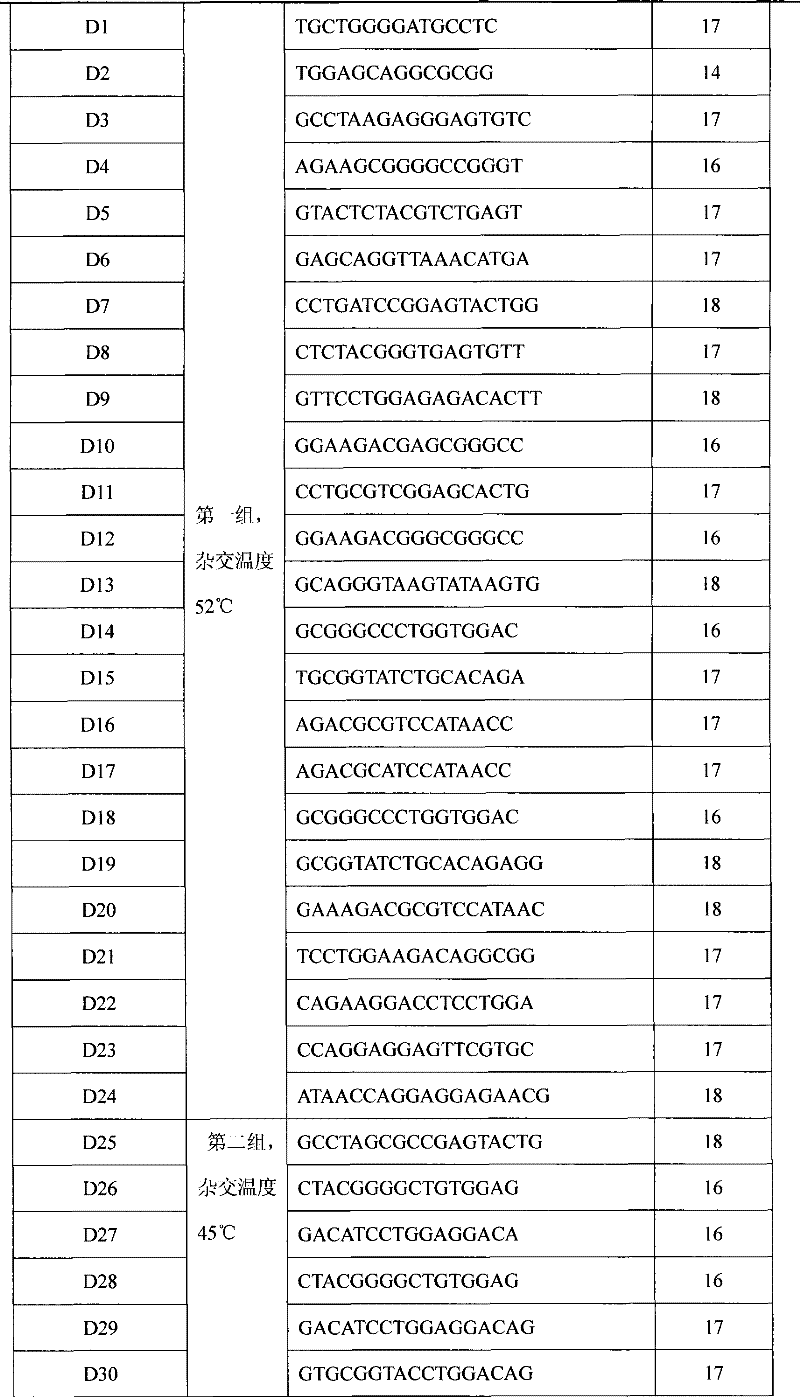
The invention relates to a gene detecting kit used for clinical detection, in particular to the kit which adopts a DNA micro-array chip and a dual-temperature hybridization probe to execute the detection and typing on human leukocyte antigen gene in a high-throughput, high-efficiency and high-specificity manner and the usage thereof.
Owner:DAAN GENE CO LTD
Primer group, kit and method for HLA (human leukocyte antigen) gene amplification and gene parting
ActiveCN108441547AReduced integrity requirementsLow costMicrobiological testing/measurementDNA/RNA fragmentationHLA-B geneHla genes
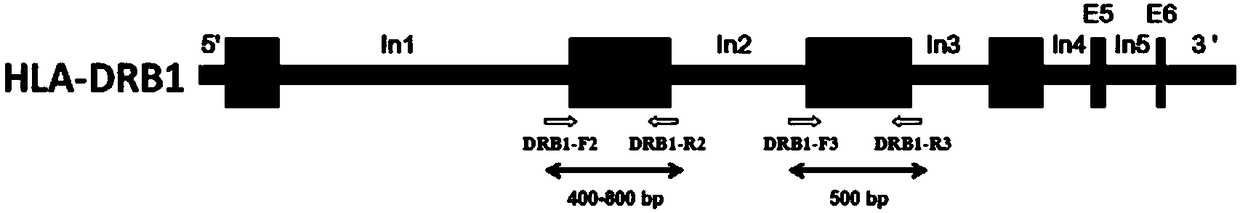
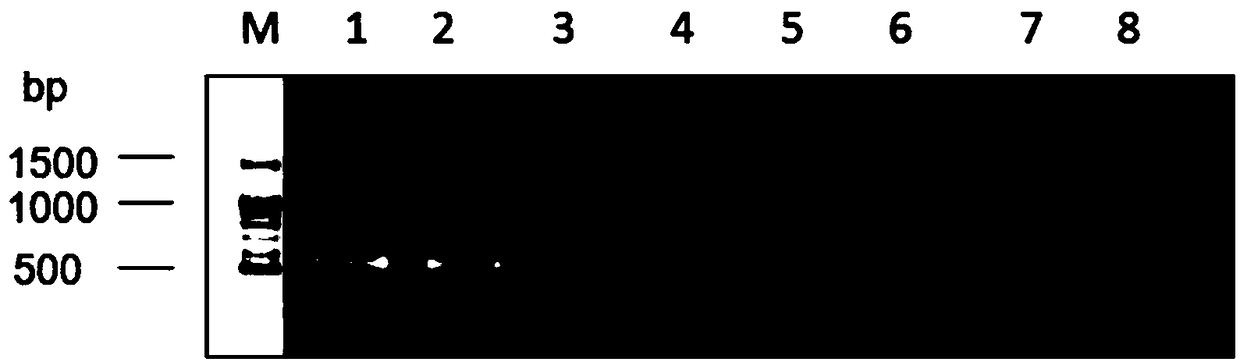
The invention provides a primer group, a kit and a method for HLA (human leukocyte antigen) gene amplification and gene parting and belongs to the field of gene detection. According to the primer group for HLA gene amplification, primers are designed according to 8 exon zones of an HLA-A gene, 8 exon zones of an HLA-B gene and an exon 2 and an exon 3 of an HLA-DRB1 gene, PCR amplification is performed on the HLA gene by use of the primers, and a product with a gene segment length larger than 400 bp and smaller than 1.5 kb is obtained. By means of the PCR amplified HLA genes, the gene segment length of the amplification product is larger than 400 bp and smaller than 1.5 kb, the requirement for completeness of a template is reduced, the amplification efficiency is high, the amplification reaction time is short, the cost is reduced, and the demands of large-scale amplification or gene parting of sample HLA genes can be met.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
Kit for predicting leukocyte antigen genotypes of the Han people by using single-nucleotide polymorphisms
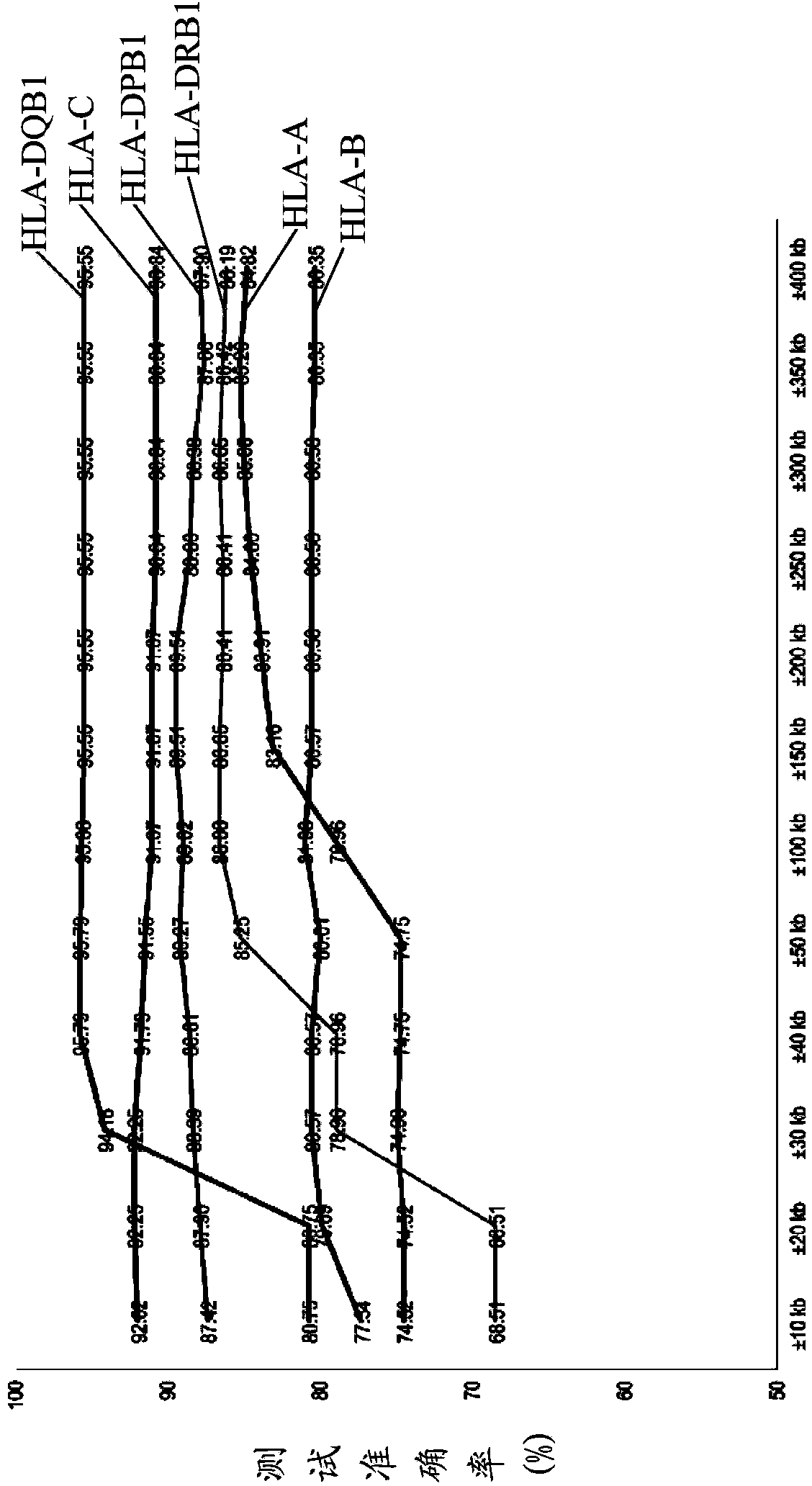
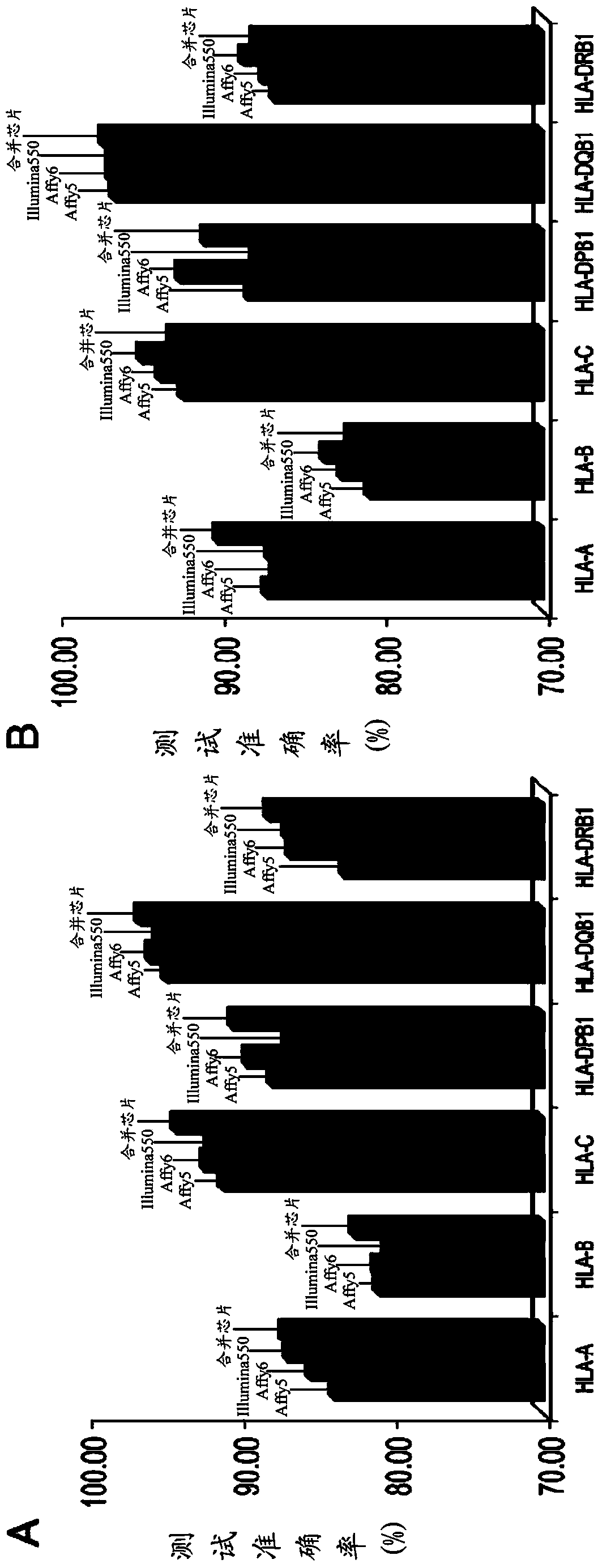
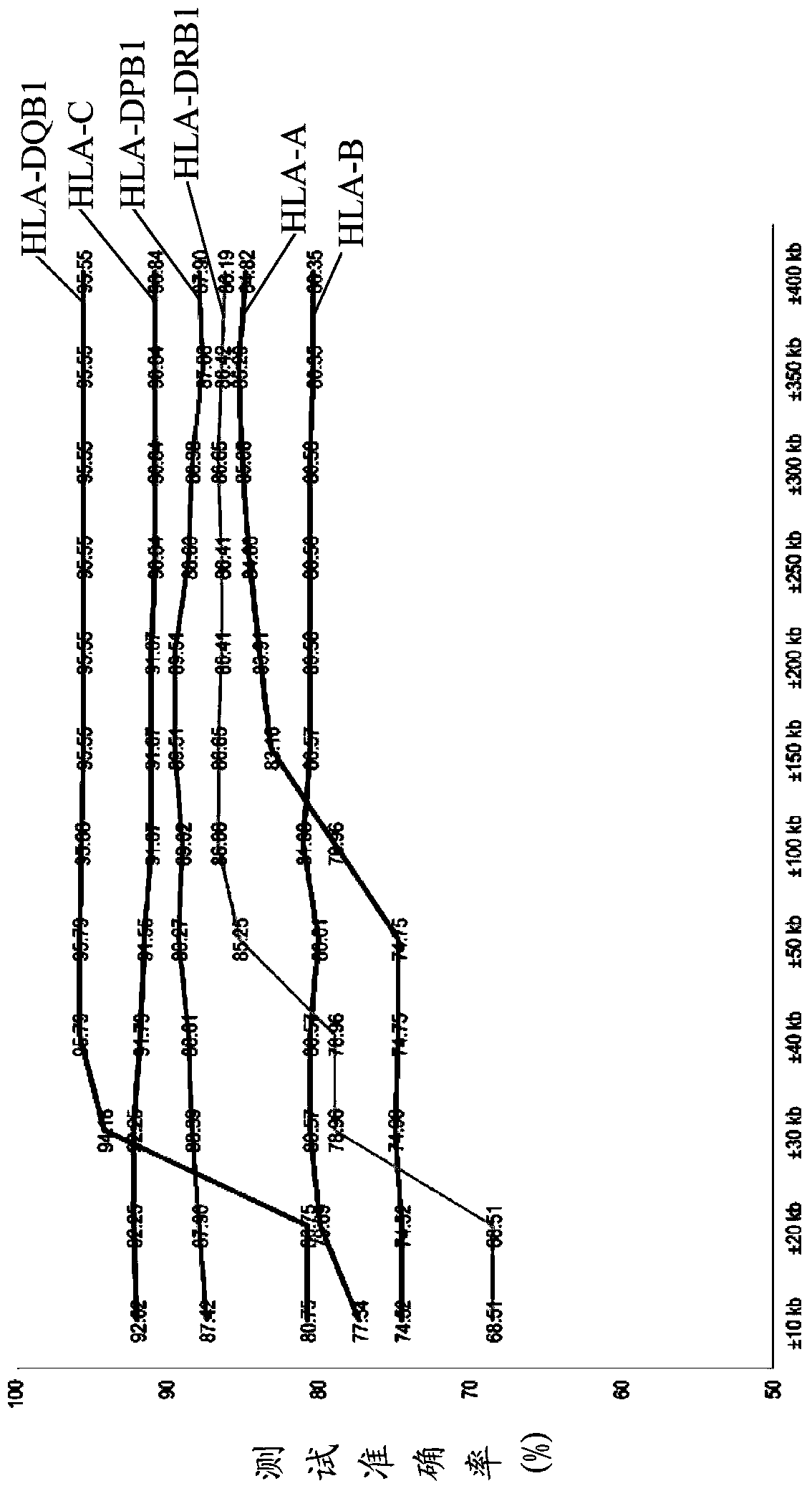
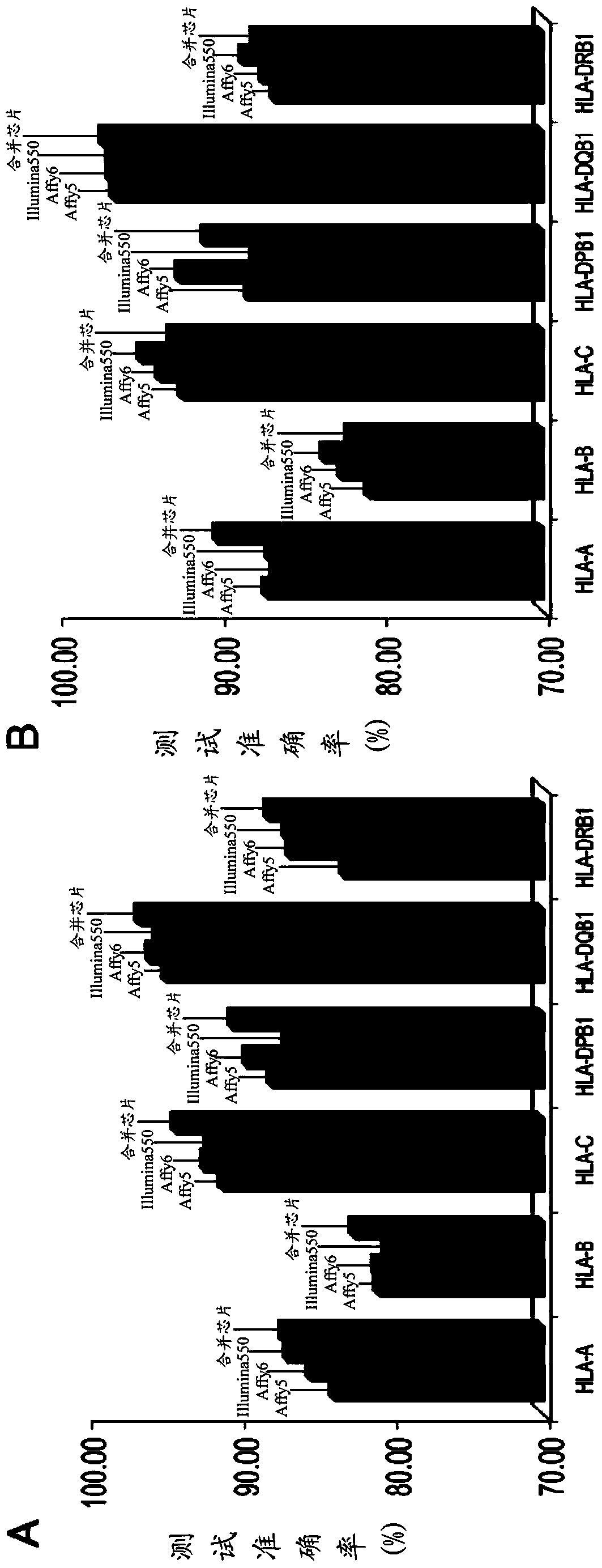
The invention discloses a kit for predicting leukocyte antigen genotypes of the Han people by using single-nucleotide polymorphisms and applications thereof. Models are predicted for the human leukocyte antigen genotypes (HLA-A, HLA-B, HLA-C, HLA-DPB1, HLA-DQB1 and HLA-DRB1) of the Asians, and the prediction results achieve optimization of the models. The typical human leukocyte antigen alleles types of the Asians are predicted according to the fixed genotypes of the single-nucleotide polymorphisms with a high accuracy ranging from 80.37% (HLA-B) to 95.79% (HLA-DQB1). Besides, the kit for predicting the leukocyte antigen alleles types by using the genotypes can save a considerable amount of time and cost.
Owner:ACAD SINIC +2
Juno<TM>-based safety medication detection kit for children and chip
InactiveCN108728524AGood curative effectReduce the risk of adverse reactionsMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCYP2C9
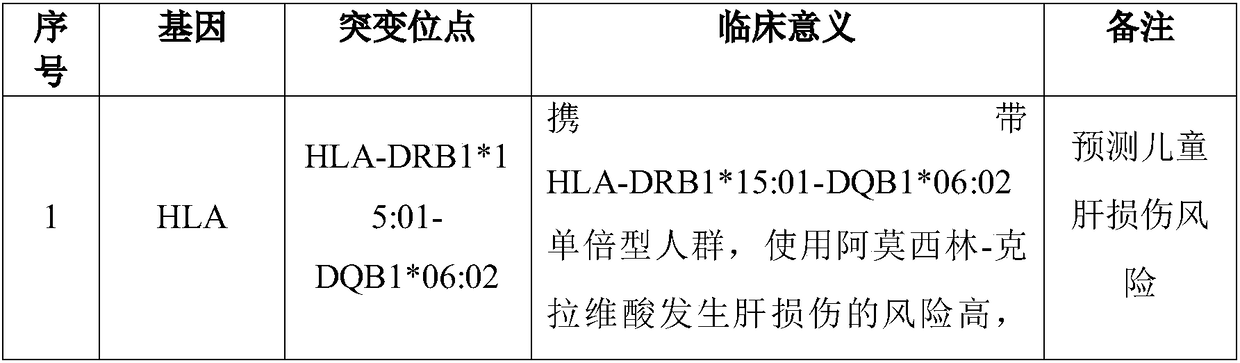
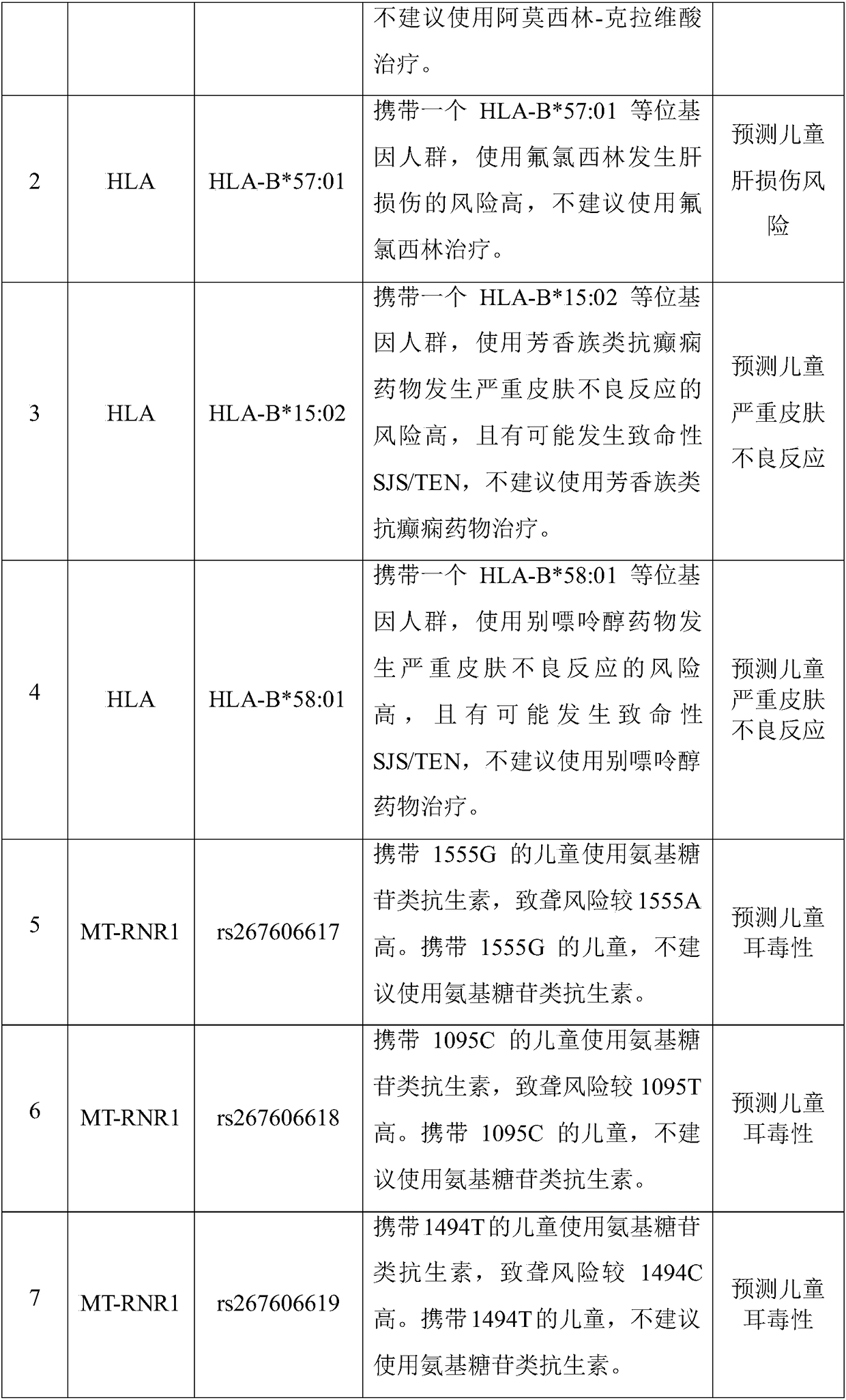
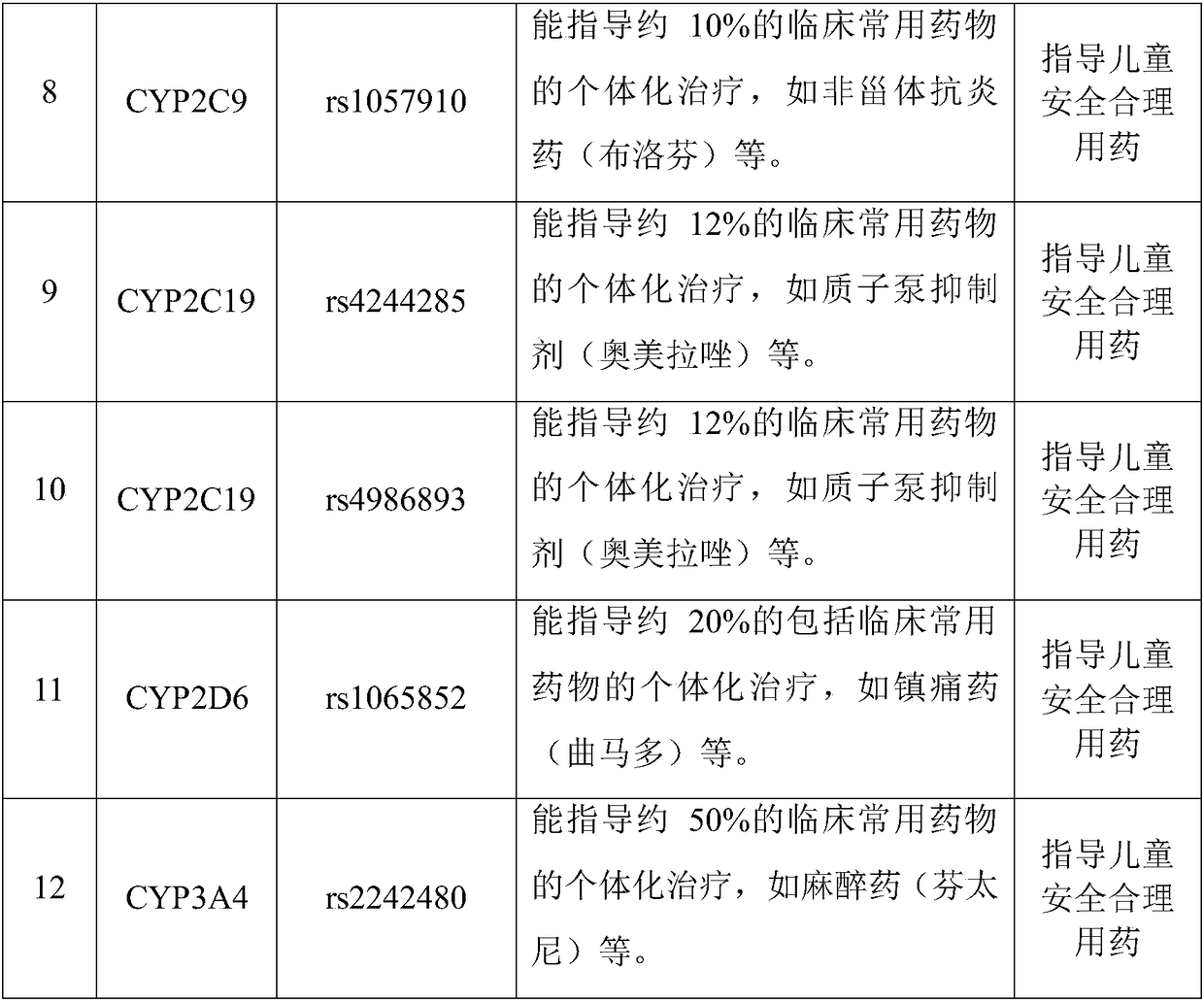
The invention discloses a Juno<TM>-based safety medication detection kit for children and a chip. The kit and the chip comprise primers at the following loci: HLA-DRB1*15:01-DQB1*06:02 loci of an HLAgene, an HLA-B*57:01 locus of the HLA gene, an HLA-B*15:02 locus of the HLA gene, an HLA-B*58:01 locus of the HLA gene, a rs267606617 locus of an MT-RNR1 gene,a rs267606618 locus of the MT-RNR1 gene,a rs267606619 locus of the MT-RNR1 gene, a rs1057910 locus of a CYP2C9 gene, a rs4244285 locus of a CYP2C19 gene, a rs4986893 locus of the CYP2C19 gene, a rs1065852 locus of a CYP2D6 gene, and a rs2242480 locus of a CYP3A4 gene.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Cervical cancer susceptibility gene typing detection kit and application thereof
The invention discloses a cervical cancer susceptibility gene typing detection kit and an application thereof. With the application of the cervical cancer susceptibility gene typing detection kit, polymorphic sites of genes HLA-DPB2, GSDMB / GSDML and HLA-DRB1, related to cervical cancer, are detected, so that genetic factors of individuals in the aspect of the cervical cancer can be understood and relative attack risks of the cervical cancer can be assessed; therefore, the kit has positive significance for early diagnosis, early treatment and early intervention of the cervical cancer. Meanwhile, the kit can also assist diagnosis of the cervical cancer, guide reasonable use of drugs, bring about benefits for predicting a risk of suffering from the disease (the cervical cancer) in offspring and the like.
Owner:SHANGHAI YIRUN BIOLOGICAL TECH
Rapid amplification diagnostic kit and amplification method for HLA (human leucocyte antigen) high-resolution genetic locus
InactiveCN108570495AStrong specificityImprove accuracyMicrobiological testing/measurementSingle strand dnaHuman leukocyte antigen
The invention discloses a rapid amplification diagnostic kit and amplification method for HLA (human leucocyte antigen) high-resolution genetic locus. The kit comprises DNA polymerase and an amplification primer; the HLA high-resolution genetic locus is HLA-DRB1, having a template sequence shown as in sequence table SEQ ID NO: 1; the amplification primer is used for performing linear index PCR (polymerase chain reaction) amplification; an upstream sequence of the amplification primer is shown as in sequence table SEQ ID NO: 2, and a downstream sequence of the amplification primer is shown as in sequence table SEQ ID NO: 3. A target fragment amplification method employing linear index amplification has the advantages that the amplification primer that is designed precisely has high synergistic efficiency and low mismatching rate, amplification efficiency is greatly improved, single-stranded DNA can be amplified directly to serve for pyrosequencing, performing marking and dual-strand separation on an amplification product is not required, complex experimental steps are omitted, and damage of strong-basicity reagents to amplified fragments is lessened.
Owner:新开源博畅(武汉)生物科技有限公司
Method for activating helper T cell
The present invention relates to a method for activating helper T cells, which includes the step of activating helper T cells by adding a WT1 peptide to antigen presenting cells, wherein the WT1 peptide has the ability to bind to an MHC class II molecule selected from HLA-DRB1*08:02 molecule, an HLA-DRB1*13:02 molecule, an HLA-DRB1*14:03 molecule, an HLA-DRB1*14:05 molecule, an HLA-DQB1*03:02 molecule, and an HLA-DQB1*04:01 molecule.
Owner:INT INST OF CANCER IMMUNOLOGY INC
WT1-origin HLA-DR-binding antigen peptide
The present invention provides a WT1-derived HLA-DRB1*0405-binding antigen peptide, a polynucleotide encoding said peptide, a helper T cell inducer comprising said peptide or polynucleotide, and the like. It is related to a partial peptide consisting of 10 - 25 contiguous amino acids in the amino acid sequence of human WT1 shown in SEQ ID NO: 1, which binds to HLA-DRB1*0405 and induces helper T cells, a polynucleotide encoding said peptide, or a helper T cell inducer comprising said peptide or polynucleotide.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Wt1-origin HLA-DR-binding antigen peptide
Owner:INT INST OF CANCER IMMUNOLOGY INC
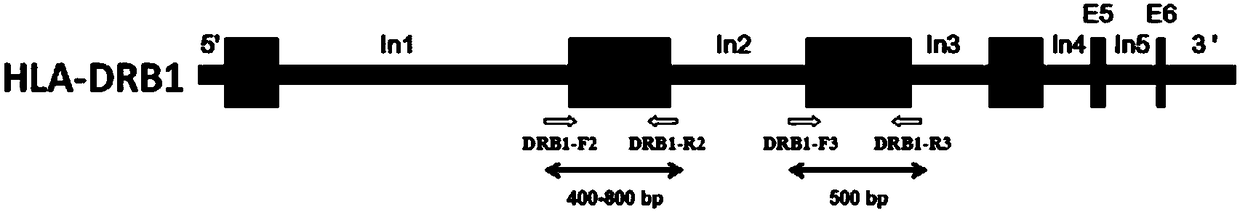
Primer group, kit and method for HLA-DRB1 gene high-resolution typing
ActiveCN108588205AShorten the lengthReduced integrity requirementsMicrobiological testing/measurementDNA/RNA fragmentationTypingOrgan transplantation
The invention discloses a primer group, kit and method for HLA-DRB1 gene typing, and belongs to the field of gene detection. According to the primer group for HLA-DRB1 gene high-resolution typing, primers are designed correspondingly according to exons 2 and exons 3 of HLA-DRB1 genes, and the primers are utilized to conduct PCR amplification on the HLA-DRB1 genes. The primers conduct PCR amplification on the HLA-DRB1 genes, thus the gene fragment length of an amplified product is smaller than 1 kb, the requirement for the integrity of a template is reduced, the amplification efficiency is high, the amplification reaction time is short, the cost is reduced, the gene typing requirement of the large-scale sample HLA-DRB1 genes can be met, the more accurate basis is provided for clinical HLA matching, a proper transplant donor is provided for a patient, the rejection reaction in the transplant process is reduced, and the success rate of organ transplantation and the survival rate of the patient are increased.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
Reagent kit for parting detection of HLA-DRB1 gene
ActiveCN101314790BHigh detection throughputStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationAntigenDNA Microarray Chip
The invention relates to a gene detecting kit used for clinical detection, in particular to the kit which adopts a DNA micro-array chip and a dual-temperature hybridization probe to execute the detection and typing on human leukocyte antigen gene in a high-throughput, high-efficiency and high-specificity manner and the usage thereof.
Owner:DAAN GENE CO LTD
Method and kit for prognosis and guiding treatment of patients with laryngeal papilloma
ActiveCN107828889AAccurate detectionReduce in quantityMicrobiological testing/measurementDNA/RNA fragmentationMedicineGene
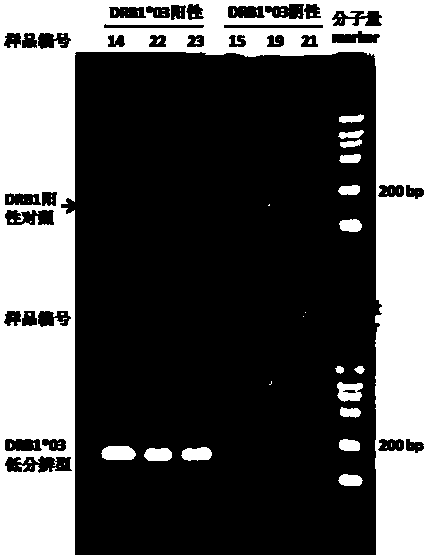
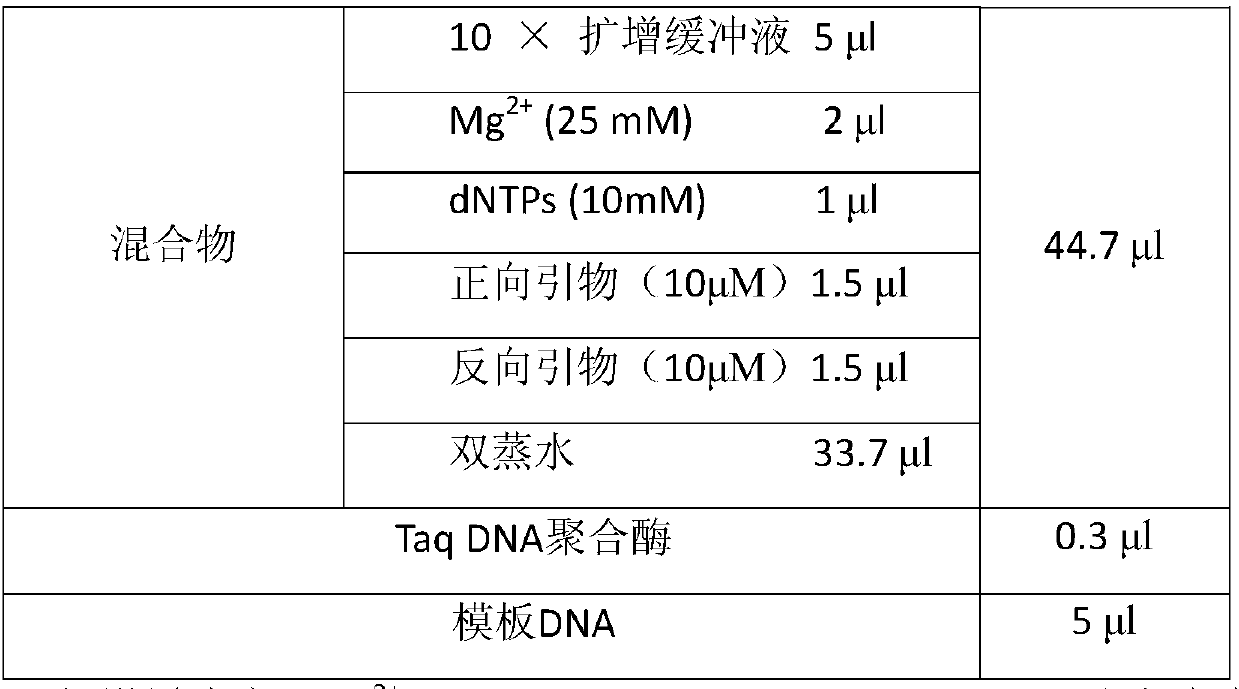
The invention relates to a gene detection method and kit capable of being used for clinical detection. The method is aimed at HLA-DRB1*03 low-resolution gene and can realize accurate, rapid, simple and inexpensive detection, and can be used for providing important information for treatment and prognosis of clinically relevant diseases such as recurrent laryngeal papilloma and the like.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Kit for predicting Chinese leukocyte antigen genotype using single nucleotide polytype
The invention discloses a kit for predicting leukocyte antigen genotypes of the Han people by using single-nucleotide polymorphisms and applications thereof. Models are predicted for the human leukocyte antigen genotypes (HLA-A, HLA-B, HLA-C, HLA-DPB1, HLA-DQB1 and HLA-DRB1) of the Asians, and the prediction results achieve optimization of the models. The typical human leukocyte antigen alleles types of the Asians are predicted according to the fixed genotypes of the single-nucleotide polymorphisms with a high accuracy ranging from 80.37% (HLA-B) to 95.79% (HLA-DQB1). Besides, the kit for predicting the leukocyte antigen alleles types by using the genotypes can save a considerable amount of time and cost.
Owner:ACAD SINIC +2
Method for detecting the susceptibility to develop adverse side effects related to bioimplants
InactiveUS20130096024A1Microbiological testing/measurementLibrary screeningMajor histocompatibilitySide effect
An in vitro method for the analysis of the genetic predisposition of an individual to develop adverse effects related to non-metallic materials implanted into the body. The method comprises determining if in a biological sample from an individual there is / are present the antigen / s HLA-B*08 and / or HLA-DRB1*03 of the major histocompatibility complex. The use of a kit for carrying out the determination and the use of the antigens as genetic markers is also disclosed.
Owner:FUNDACIO HOSPITAL UNIVERSITARI VALL DHEBRON INST DE RECERCA
Method for measuring immunogenicity of protein agent
InactiveUS20190194618A1Improve accuracyImprove efficiencyCompound screeningApoptosis detectionDendritic cellGenotype
A method for determining immunogenicity of a protein agent. The method includes constructing a library of peripheral blood mononuclear cells having various HLA-DRB1 genotypes; culturing peripheral blood mononuclear cell CD14+ monocyte-derived immature dendritic cells for each genotype in a medium containing a protein to be measured, GM-CSF, IL-4, TNF-α, IL-1β, IL-6 and PGF2 to prepare mature dendritic cells; removing CD8+ T cells from the peripheral blood mononuclear cells for each genotype to prepare CD8+ T cell-free peripheral blood mononuclear cells; co-culturing the mature dendritic cells and the CD8+ T cell-free peripheral blood mononuclear cells at a cell count ratio of approximately 1:5 to 1:20; and quantifying the CD4+ T cells proliferated by co-cultivation per genotype.
Owner:MOGAM INST FOR BIOMEDICAL RES
dna typing method and kit for hla gene
ActiveCN103890190BHigh precisionAllele novelMicrobiological testing/measurementRecombinant DNA-technologyHuman DNA sequencingClassification methods
Owner:GENODIVE PHARMA
Detection method for predicting H7N9 susceptible populations based on HLA gene polymorphism sites and application of detection method
ActiveCN109554460AImprove reliabilityImprove effectivenessMicrobiological testing/measurementDNA/RNA fragmentationDiseaseSmall sample
The invention discloses a detection method for predicting H7N9 susceptible populations based on HLA gene polymorphism sites. The detection method is characterized by comprising the following steps ofthe step for collecting A(H7N9) patients and healthy contrast specimens; the step of prescreening HLA candidate sites; and the step of performing verification and validation on HLA high-risk sites. The invention further discloses an application for predicting H7N9 susceptible populations based on HLA gene polymorphism sites. The application is characterized in that the polymorphism sites compriseHLA-A*02:06 and / or HLA-DRB1*12:02. A multiplex PCR target gene catching and high-flux bibasic sequencing united technique is adopted for detection and analysis on gene polymorphism of A(H7N9) avian influenza patients and healthy contrast HLA regions. Other researched large sample health contrast is used for verification, the reliability of research results of small sample group genome is improved,and the associated validity of the screened gene sites and diseases is improved.
Owner:SHANGHAI MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION +2
Application of HLA (human leukocyte antigen) gene to judging white vulval lesions of Chinese han-nationality women
InactiveCN104774946AExplore correlationMicrobiological testing/measurementGenetic engineeringImmunogeneticsHLA-B
The invention discloses application of an HLA (human leukocyte antigen) gene to judging white vulval lesions of Chinese han-nationality women, and belongs to the field of gynaecology and immunological genetics. The susceptible population of vulvar lichen sclerosus and vulvar squamous cell hyperplasia is judged by detecting and screening a susceptibility gene and a resistance gene through the HLA gene, and control study and judgment analysis are performed by observing conditions of alleles of HLA-B*5, HLA-DRB1*12, HLA-B*40, HLA-A*11, HLA-A*31, HLA-B*13, HLA-B*35, HLA-DRB1*01 and HLA-DRB1*03 of vulvar lichen sclerosus and vulvar squamous cell hyperplasia patients. The application is of important guiding significance on early-stage diagnosis, predication, prevention and treatment of vulvar dystrophy.
Owner:刘桂兰
A method for assessing hypersensitivity reactions to the antiepileptic drug phenytoin using hla alleles
A method to use HLA alleles to evaluate drug allergic reactions caused by the antiepileptic drug phenytoin, including: Stevens Johnson syndrome (SJS), toxic epidermolysis (TEN), or drug eruption combined with eosinophilia and systemic symptoms (DRESS). Gene polymorphism of CYP2C gene (including CYP2C9, CYP2C19, CYP2C8 and CYP2C18), HLA genotype (including HLA‑A*0207, HLA‑A*2402, HLA‑B*1301, HLA‑B*1502, HLA‑B *4001, HLA‑B*4609, HLA‑B*5101, HLA‑DRB1*1001 and HLA‑DRB1*1502), as well as the phenytoin concentration in the patient’s plasma may cause phenytoin to cause adverse reactions.
Owner:CHANG GUNG MEDICAL FOUND CHANG GUNG MEMORIAL HOSPITAL AT KEELUNG
Risk assessment for phenytoin-induced adverse drug reactions
Owner:CHUNG WEN HUNG +1
Application of HLA-DRB1*04:03 allele to evaluation of risk of occurrence of drug eruption caused by methimazole
The invention discloses application of HLA-DRB1*04:03 allele to evaluation of risk of occurrence of drug eruption caused by methimazole, discloses application of a material for detecting the HLA-DRB1*04:03 allele to preparation of a kit and also discloses a kit comprising the material for detecting the HLA-DRB1*04:03 allele. The kit has the functions of (a) evaluating the risk of occurrence of drug eruption caused by methimazole of testees, (b) evaluating the risk of occurrence of drug eruption caused by methimazole of the testees and showing that the risk of occurrence of drug eruption of thetestees carrying the HLA-DRB1*04:03 allele after taking methimazole is higher than that of the testees without carrying the HLA-DRB1*04:03 allele, (c) evaluating whether the testees are suitable fortaking methimazole and (d) evaluating whether the testees are suitable for taking methimazole and showing that the testees carrying the HLA-DRB1*04:03 allele are not suitable for taking methimazole.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com