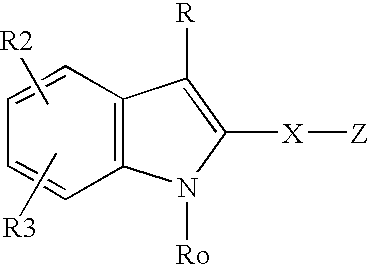
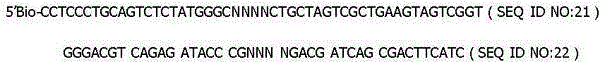Patents
Literature
37 results about "CYP2C9" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
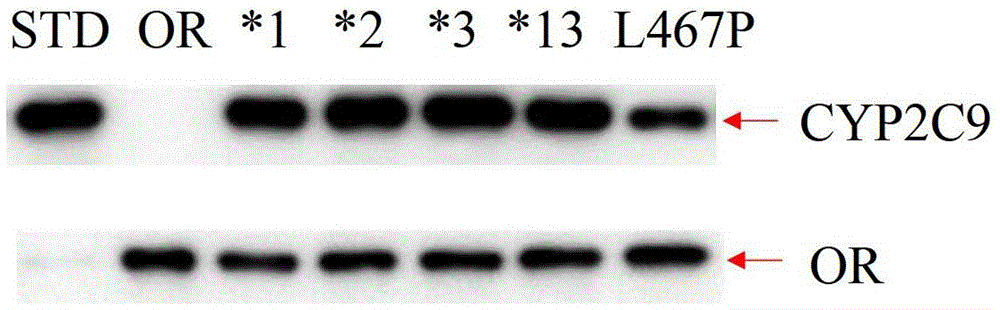
Cytochrome P450 2C9 (abbreviated CYP2C9) is an enzyme that in humans is encoded by the CYP2C9 gene.
Method and composition for treating neurodegenerative disorders
InactiveUS20050288375A1Delay and slow onsetImprove the level ofBiocideNervous disorderNeuro-degenerative diseaseCYP2C9
The invention provides compositions and methods for treating neurodegenerative disorders. A method of the invention involves administering to an individual in need of treatment a composition having an R-NSAID and an NMDA antagonist. Another method of the invention involves administering to an individual in need of treatment a composition having at least two compounds that are capable of interacting with CYP2C9, wherein at least one of said compounds is an Aβ42 lowering agent. The methods and compositions of the invention are useful for treating and preventing neurodegenerative disorders like Alzheimer's disease, dementia, mild cognitive impairment.
Owner:MYRIAD GENETICS
Medicine metabolic relevant loci detection method
InactiveCN101760528AAccurate and reliable metabolic strengthAvoid adverse reactionsMicrobiological testing/measurementDrug metabolismFluorescence
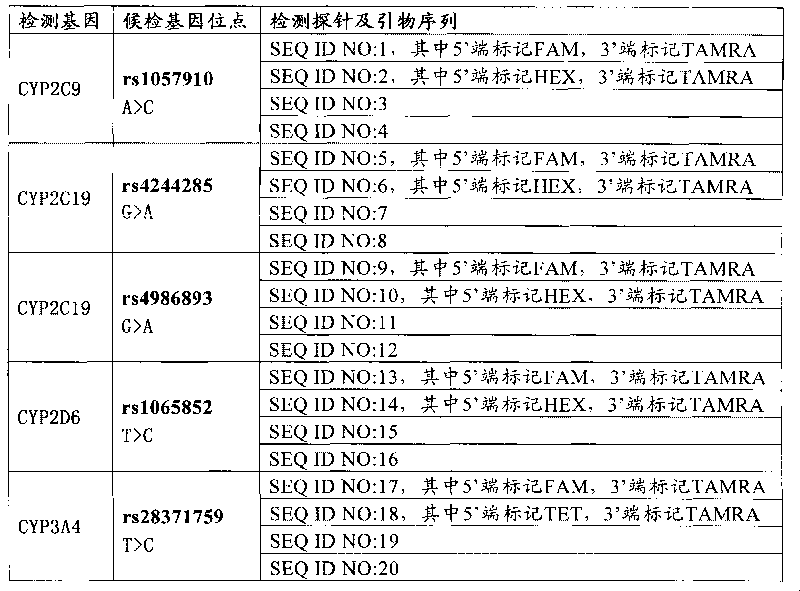
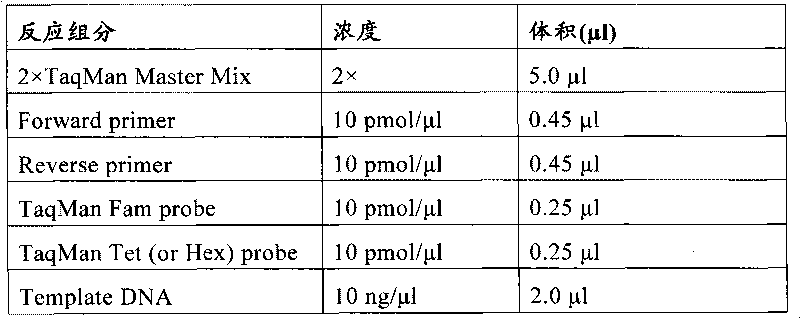
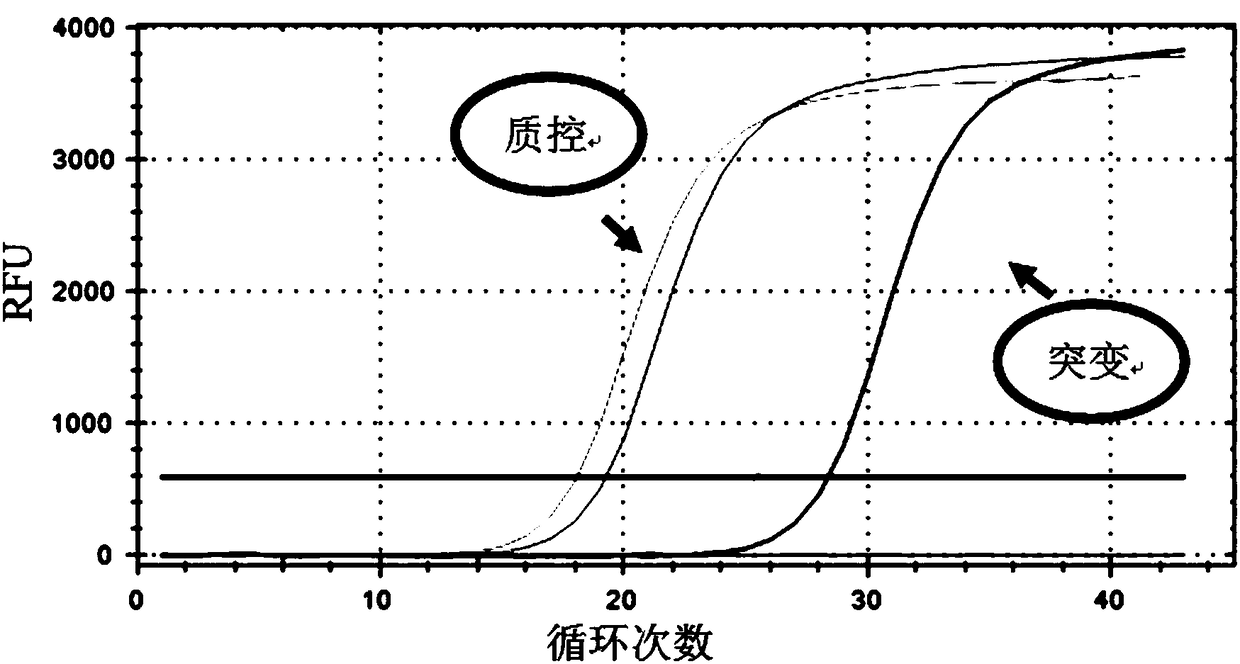
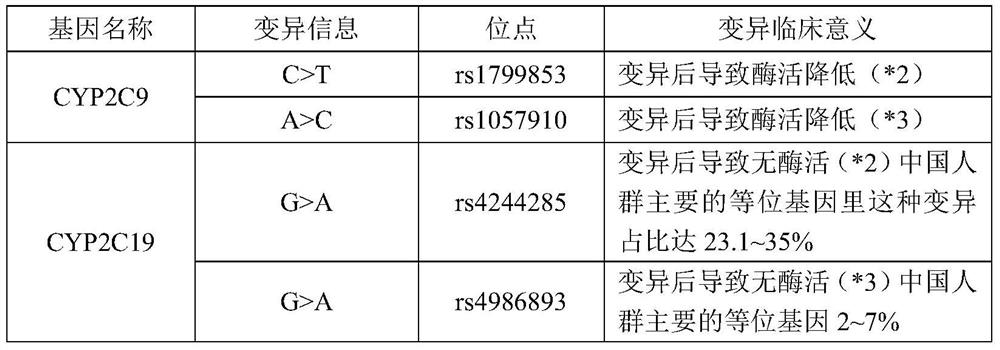
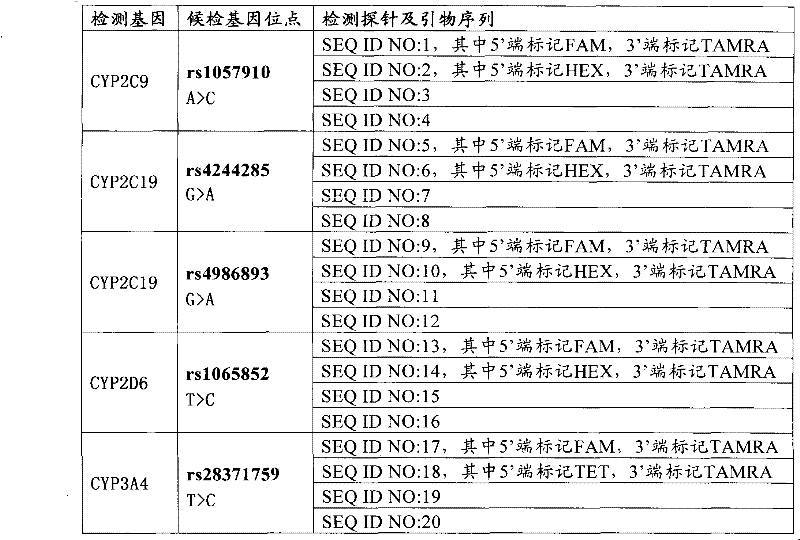
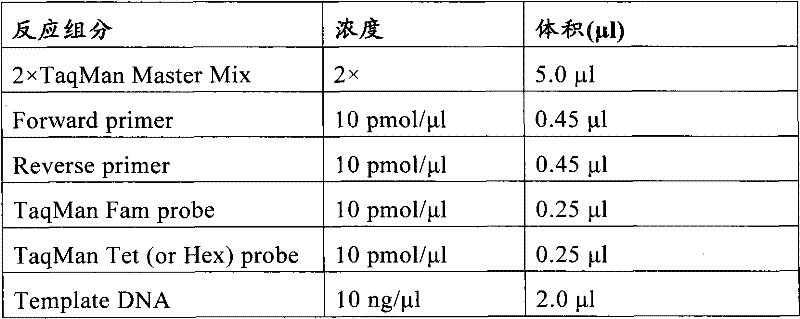
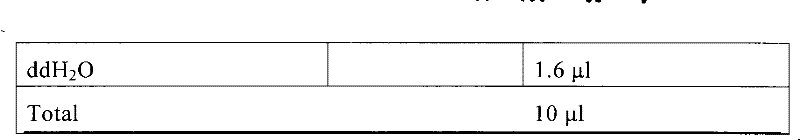
The invention relates to a medicine metabolic relevant loci detection method, which comprises the following steps: extracting genome DNA from human samples; respectively designing a Taqman probe pair and a primer pair according to at least two medicine metabolic relevant genes; respectively marking the 5' end and the 3'end of the Taqman probe pair with fluorescence reporting genes and fluorescence quenching genes; carrying out fluorescence quantitative PCR augmentation on the genome DNA; and judging whether the medicine metabolic relevant genes have the mutation according to the fluorescence quantitative PCR augmentation results. Preferably, the number of the medicine metabolic relevant genes is four, the Taqman probe pair and the primer pair are used for detecting a loci rs1057910 of a gene CYP2C9, a loci rs4244285 of a gene CYP2C19, a loci rs4986893of a gene CYP2C19, a loci rs1065852 of a gene CYP2D6 and a loci rs28371759 of a gene CYP3A4. The invention has the advantages of ingenious design, simple operation and accurate and reliable detection results, and provides the reference frame for determining whether professional doctors are needed to be consulted so as to make sure the medicine can be taken or not or the proper dosage and the like when a certain medicine is taken.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
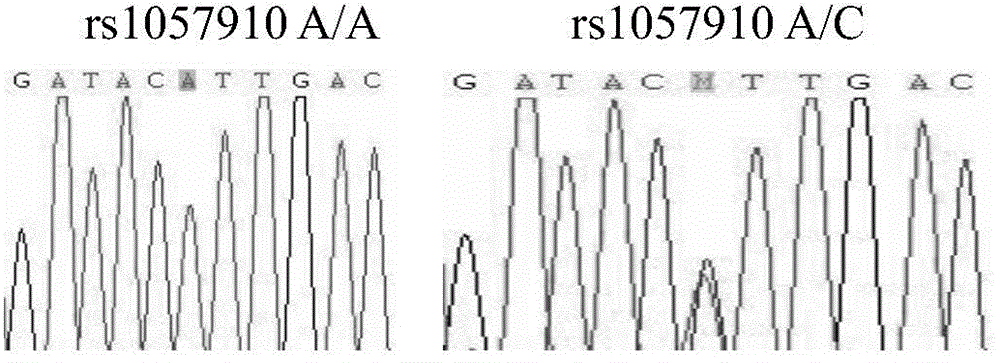
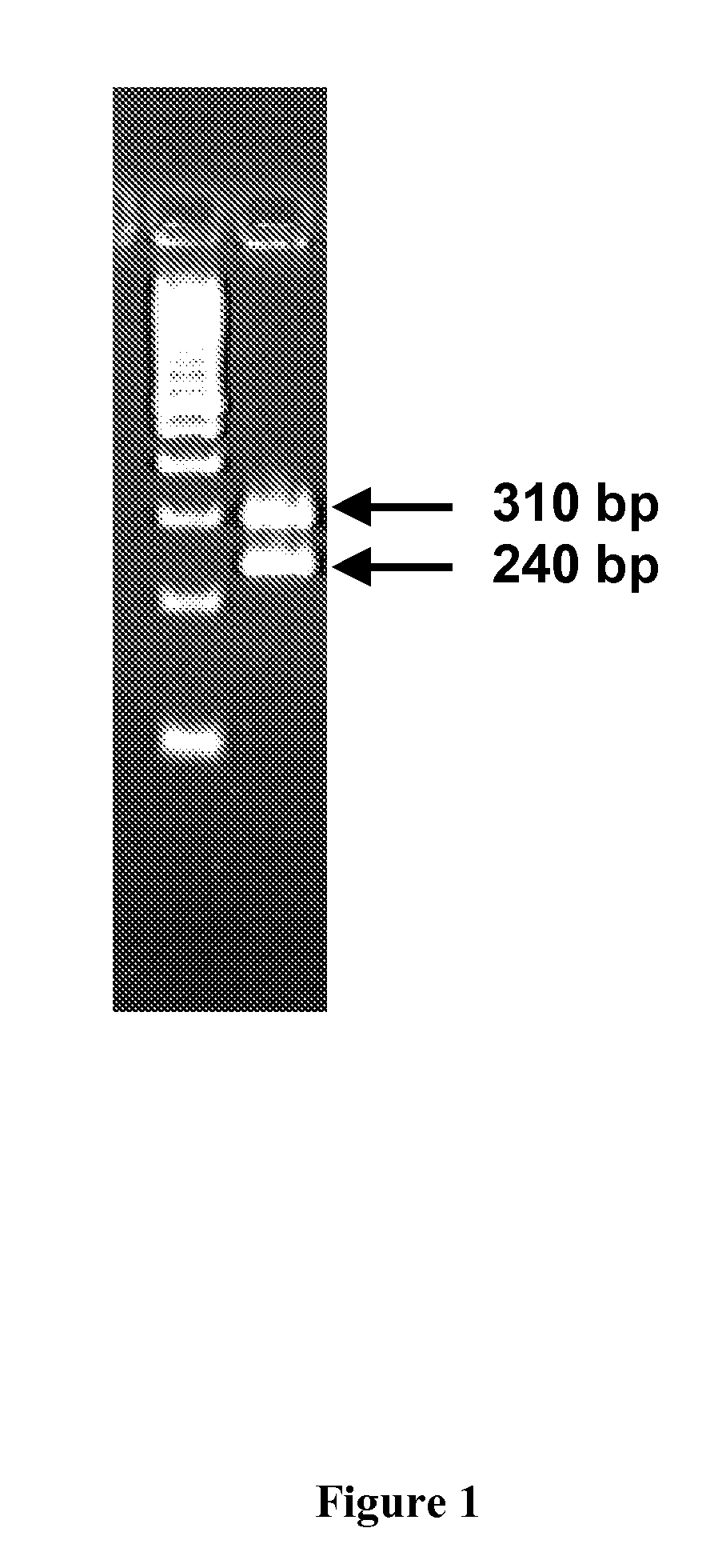
Method for detecting CYP2C9 gene exon 9 mononucleotide polymorphism
The invention discloses a detection method for the CYP2C9 gene ninth exon single nucleotide polymorphism, belonging to the technical field of the genetic engineering. The method comprises the following steps: step one, by taking the CYP2C9 gene ninth exon in a GenBank database and the exon and intron joint part sequence as a template, a pair of allele-specific nucleic acid primers are designed; the primer pair is used for amplifying the DNA sequence of the CYP2C9 gene ninth exon; and then corresponding separated nucleic acids are obtained by conducting separation and purification on the amplified product; and step two, detection is carried out on the 1739-1740 nucleotides of the CYP2C9 gene ninth exon of the separated nucleic acids so as to fix whether single nucleotide polymorphism exists or not. The invention can be used for studying the relationship between the CYP2C9 gene polymorphism in the population of China and the clinical drug safety, and provides guiding basis for the research and development of new drugs.
Owner:SHANGHAI JIAO TONG UNIV
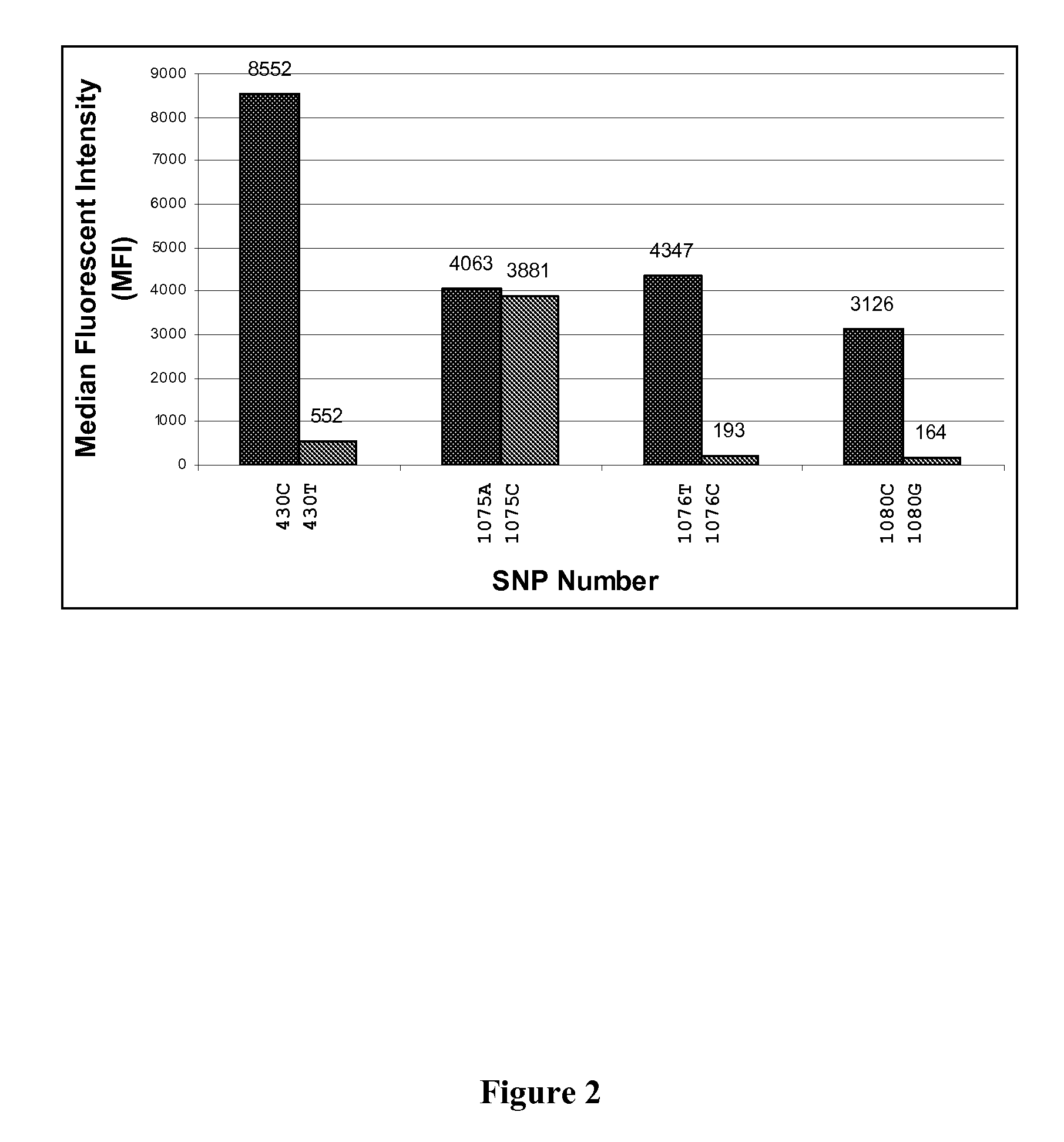
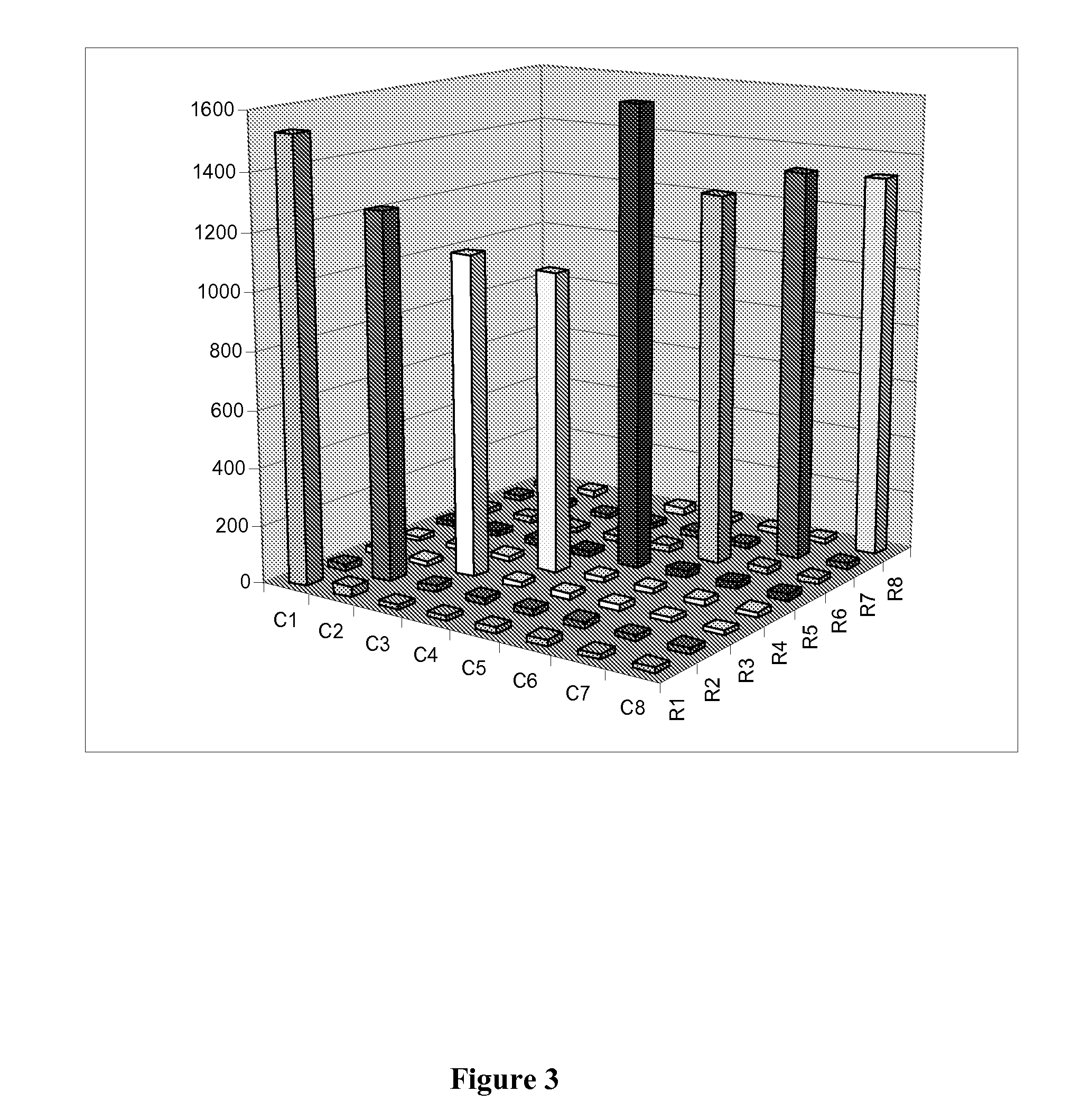
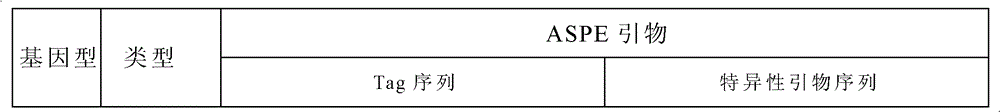
Specific primer and liquid phase chip for SNP (Single Nucleotide Polymorphism) detection of CYP2C9 (Cytochrome P4502C9)
ActiveCN102154455AImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereGenetics
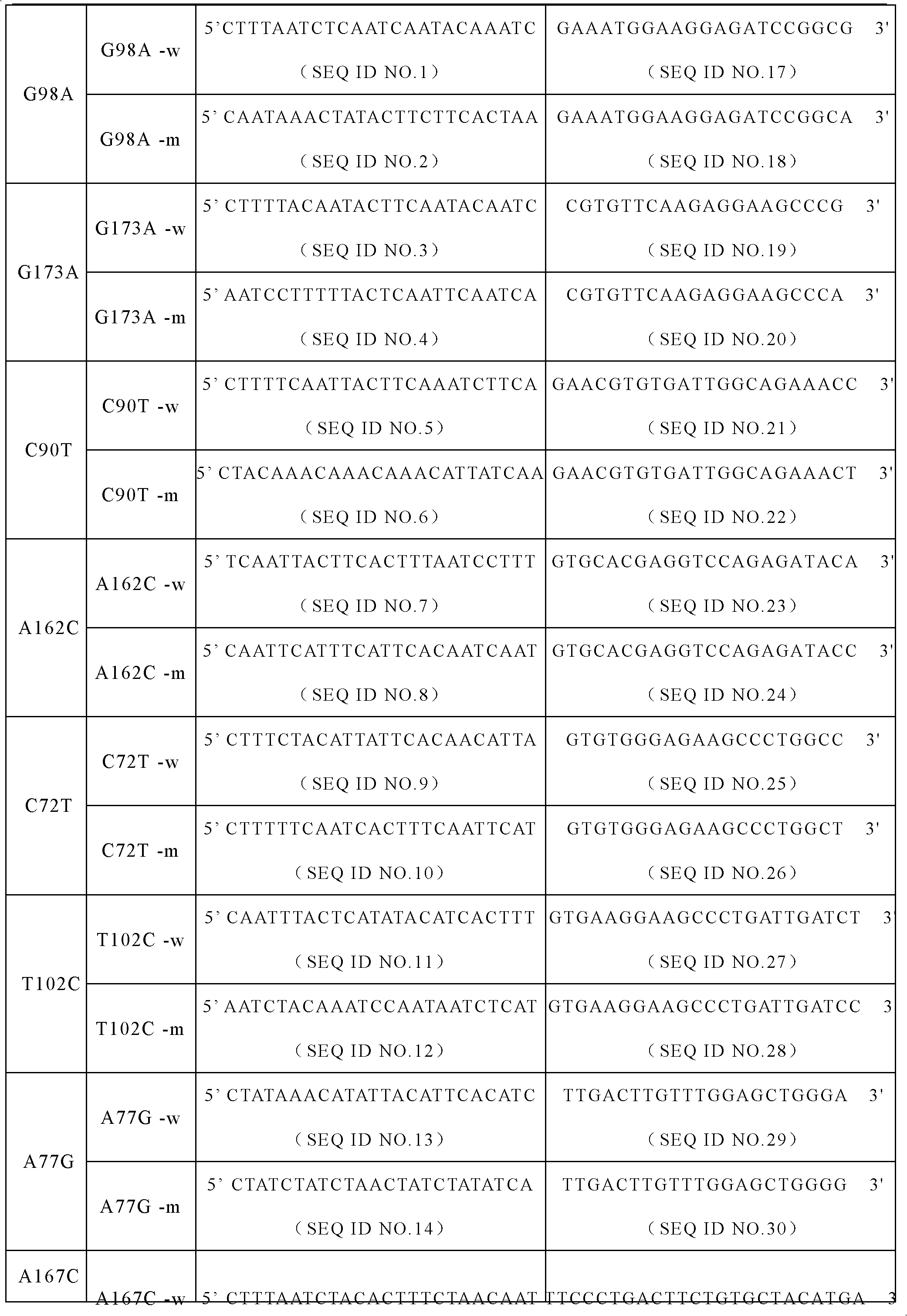
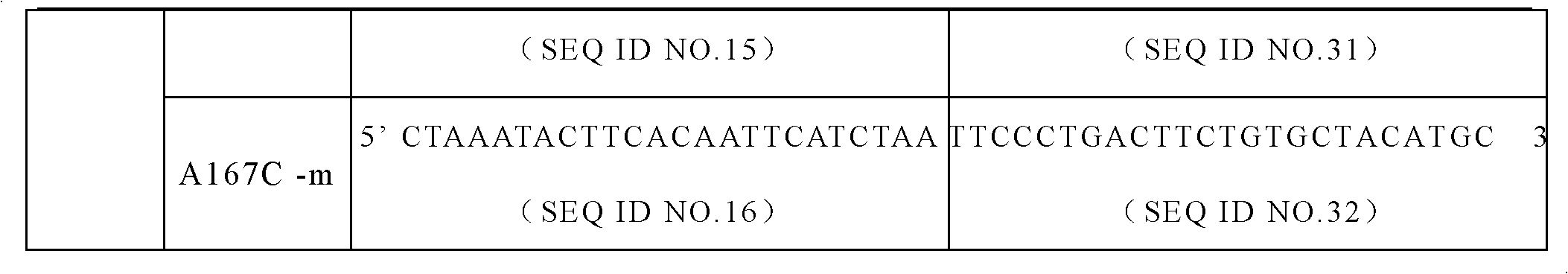
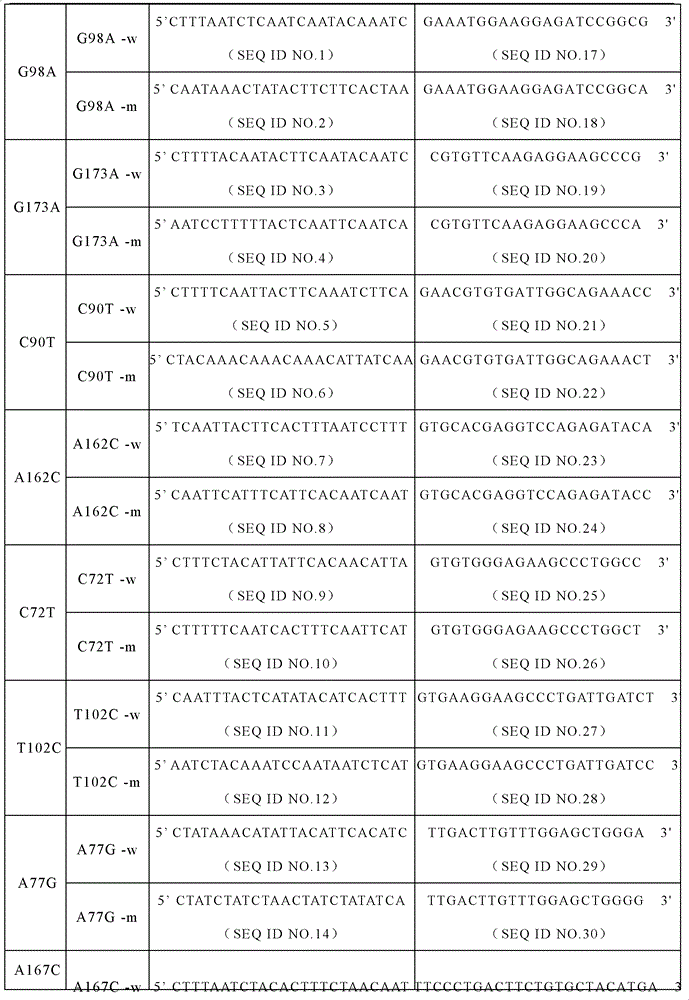
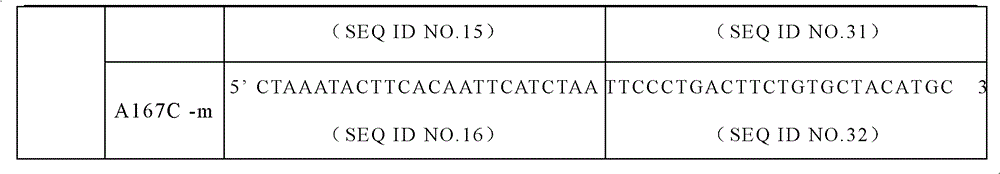
The invention discloses a specific primer and liquid phase chip for SNP (Single Nucleotide Polymorphism) detection of CYP2C9 (Cytochrome P4502C9), wherein the liquid phase chip comprises an ASPE primer consisting of a tag sequence of 5' end and a specific primer of 3' end aiming at target gene mutation, a microsphere coated by the anti-tag sequence, and an amplification primer; the specific primer includes: SEQ ID NO.17 and SEQ ID NO.18 aiming at G98A SNP locus, SEQ ID NO.19 and SEQ ID NO.20 aiming at G173A SNP locus, SEQ ID NO.21 and SEQ ID NO.22 aiming at C90T SNP locus, SEQ ID NO.23 and SEQ ID NO.24 aiming at A162C SNP locus, SEQ ID NO.25 and SEQ ID NO.26 aiming at C72T SNP locus, SEQ ID NO.27 and SEQ ID NO.28 aiming at T102C SNP locus, SEQ ID NO.29 and SEQ ID NO.30 aiming at A77G SNP locus, and / or SEQ ID NO.31 and SEQ ID NO.32 aiming at A167C SNP locus. The matching rate between a detection result obtained by adopting the liquid phase chip for SNP detection of CYP2C9 and a sequencing method can be 100%.
Owner:SUREXAM BIO TECH
SNP typing method and kit
InactiveCN106834427AImprove accuracyReduce false signalsMicrobiological testing/measurementBinding siteTyping methods
The invention relates to the technical field of DNA detection and provides an SNP typing method and kit. A to-be-tested SNP site comprises SNP sites in CYP2C9, VKORC1 and CYP2C19 genes. The SNP typing method comprises the following steps: replacing a fluorescently-labeled oligonucleotide probe with a non-fluorescently-labeled oligonucleotide probe, performing one-time connection sequencing reaction on to-be-sequenced library molecules and then obtaining sequence information of the to-be-tested SNP site through connection sequencing. The SNP typing kit comprises a CYP2C9 specific primer pair, a VKORC1 specific primer pair, a CYP2C19 specific primer pair and the non-fluorescently-labeled oligonucleotide probe. The SNP typing method and kit provided by the invention have the benefit that through sealing non-specific binding sites in the to-be-sequenced library molecules, a false signal in a result obtained by the connection sequencing is reduced, so that the accuracy of SNP site detection is improved.
Owner:GUANGZHOU KANGXINRUI GENE HEALTH TECH CO LTD
Multi-gene detection kit for anti-epileptic drug medication guidance and use method thereof
InactiveCN109825574AAvoid false negativesAvoid false positivesMicrobiological testing/measurementAntiepileptic drugMultiplex pcrs
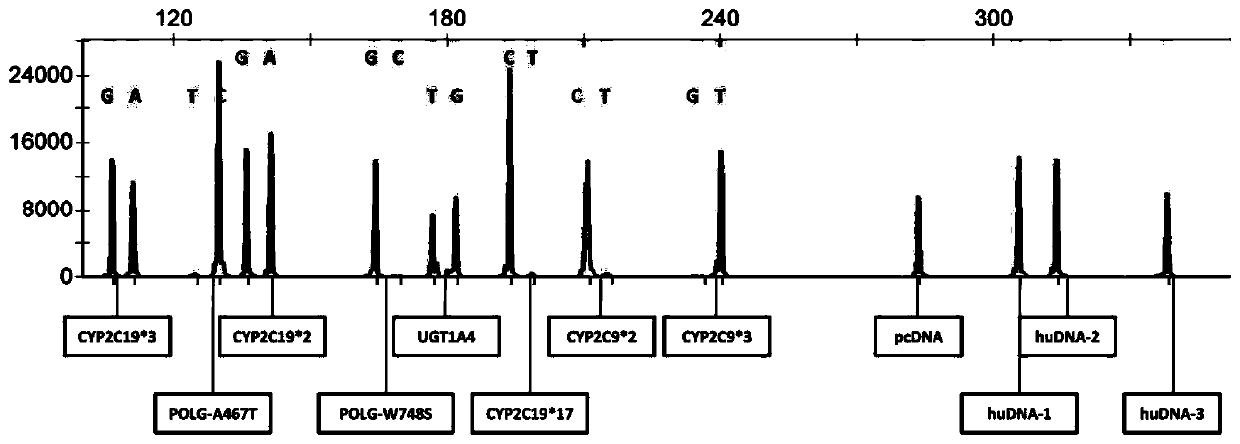
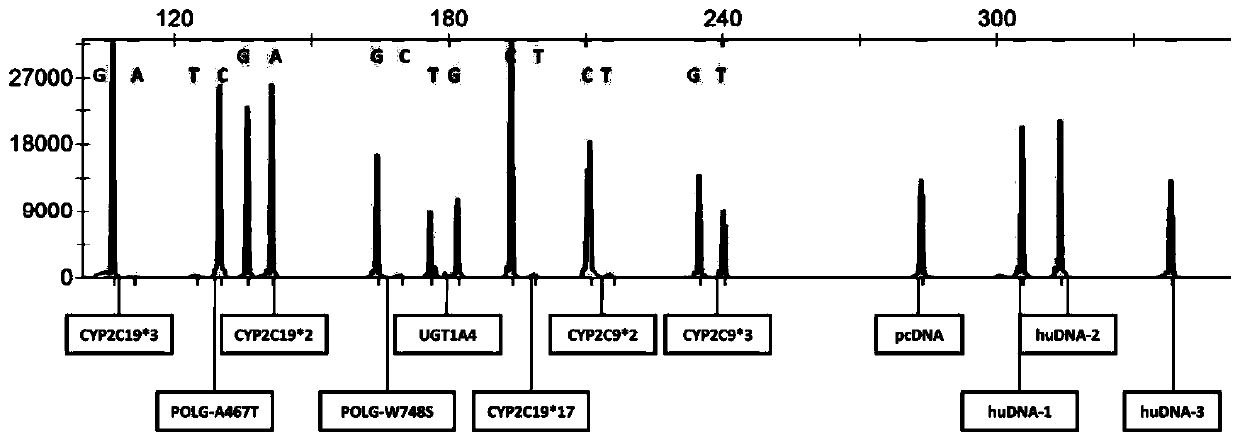
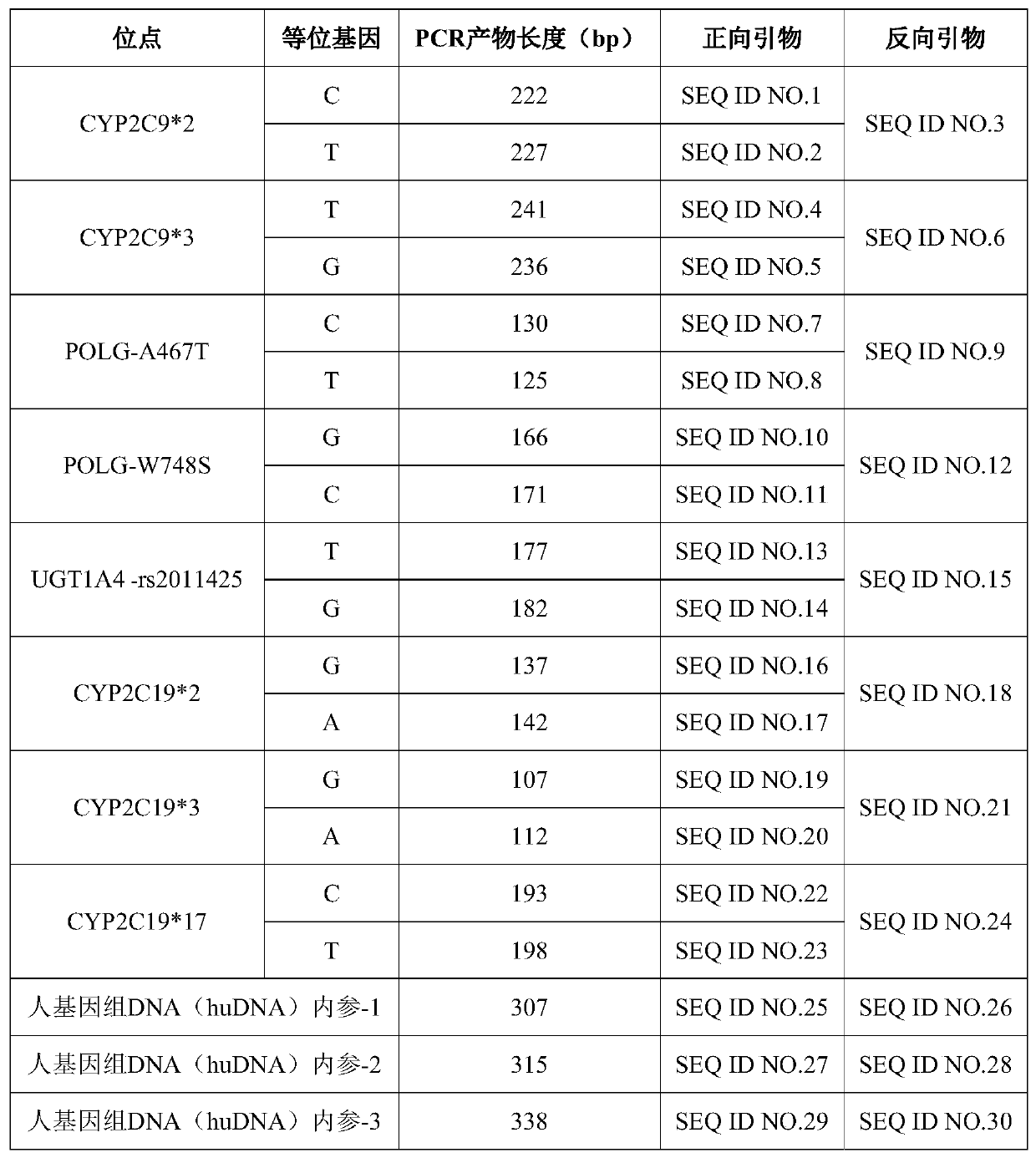
The invention discloses a multi-gene detection kit for anti-epileptic drug medication guidance and a use method thereof. A multi-PCR and fragment length / mass analysis method is used for synchronously,quickly and quantitatively detecting eight single nucleotide polymorphism (SNP) loci on four genes, including CYP2C9, CYP2C19, POLG and UGT1A4, related to anti-epileptic drug metabolism, transfer andtarget site actions. Detection includes the steps that 1, oral cast-off cells are collected and stored in a cell acquisition card or a blood sample is collected and nucleic acid is extracted; 2, thecell acquisition card or the nucleic acid in step 1 serves as a template for multi-PCR amplification; 3, according to the length / mass of PCR product fragments, the eight SNP loci, three personal genome DNA reference (huDNA) genes and a PCR product of a PCR reaction internal reference are synchronously separated; 4, results are subjected to analysis and interpretation. The kit has the advantages ofbeing quick to use, high in sensitivity, good in repeatability, high in specificity, high in flux and low in cost.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
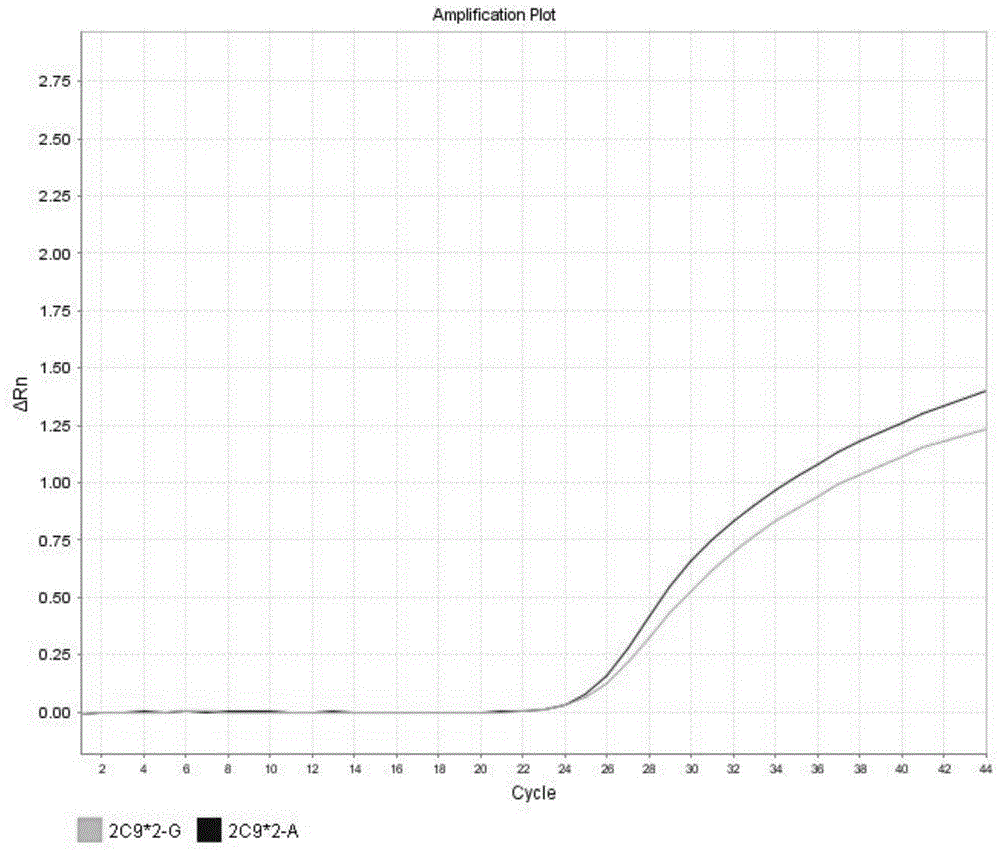
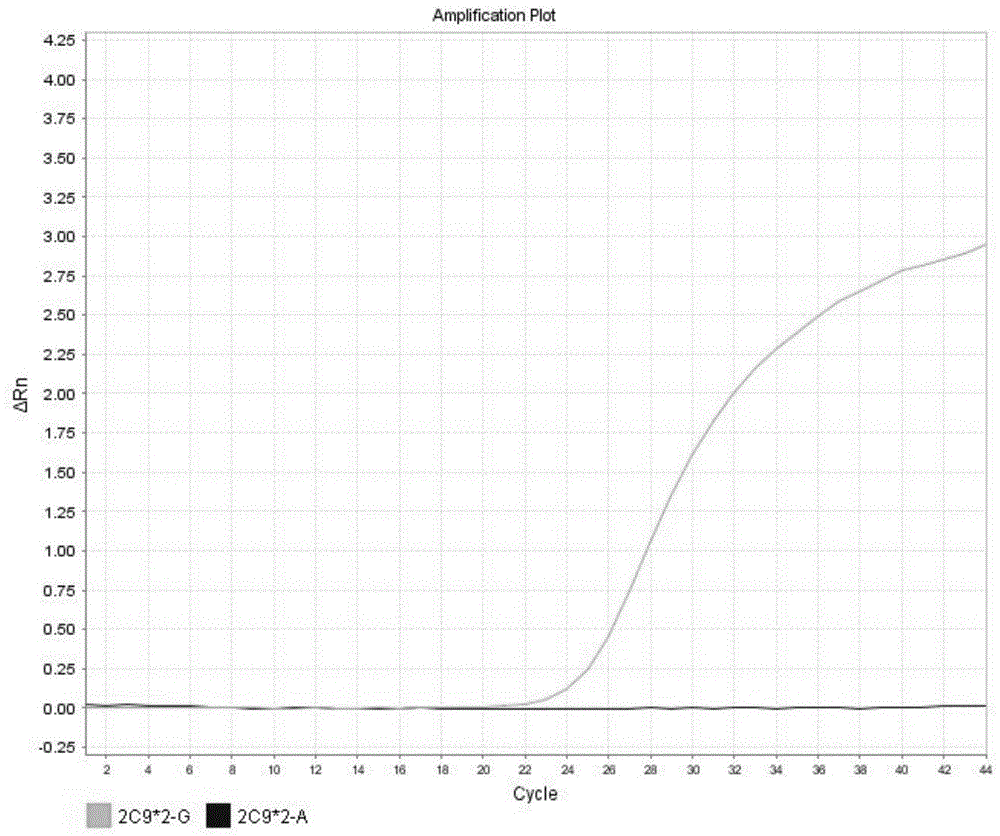
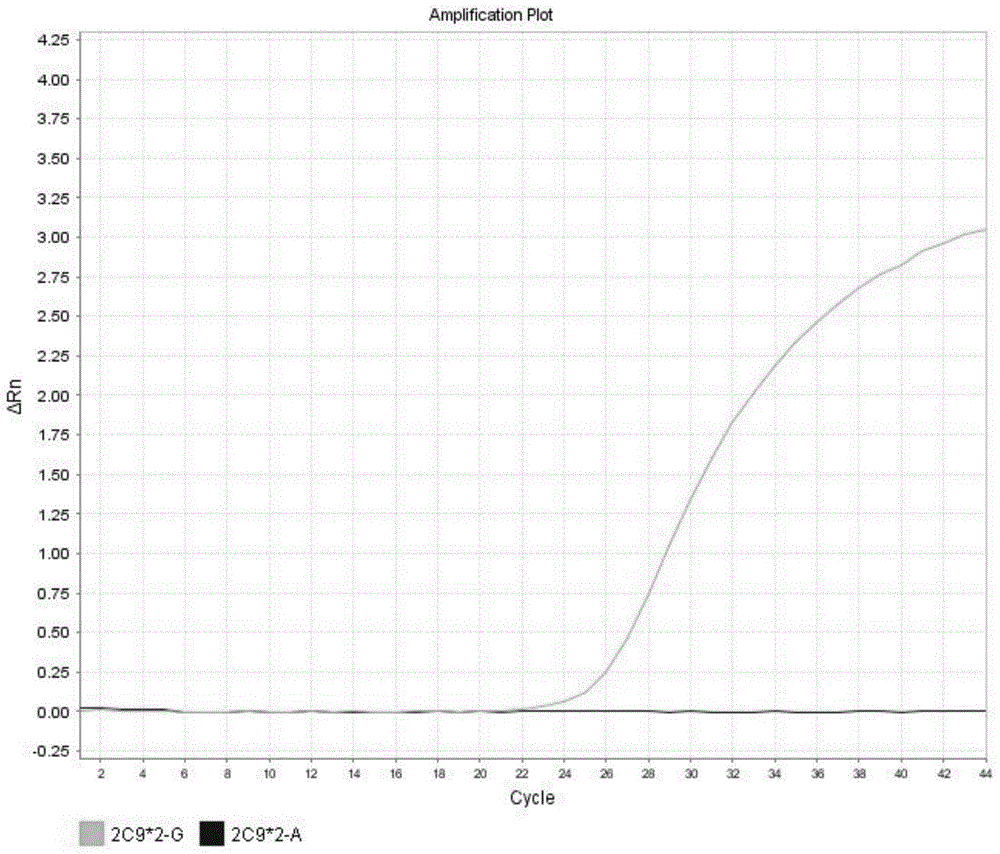
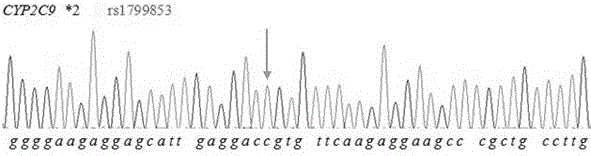
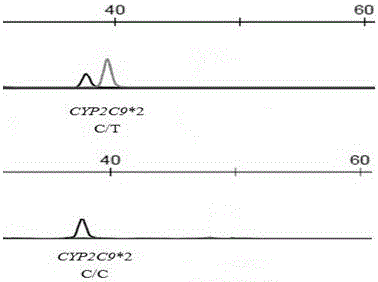
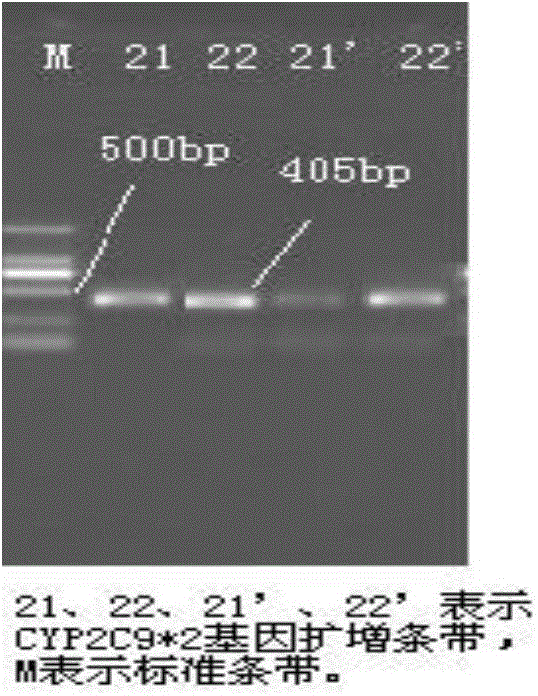
Detection method for CYP2C9*2 gene polymorphism, as well as nucleic acid probe and kit for method
InactiveCN104561300AQuick and easy analysisEasy to analyzeMicrobiological testing/measurementDNA/RNA fragmentationForward primerFluorescence
The invention discloses a detecting probe for CYP2C9*2 gene polymorphism. The nucleotide sequences of the detecting probe are selected from a sequence 1 and a sequence 2; a fluorescent group and a quenching group are respectively arranged at the ends (5'and 3'); the peak values of the wavelengths of the florescent light emitted from the fluorescent group are at different positions. Preferably, the quenching group is selected from MGB and BHQ2; preferably, the fluorescent group is selected from FAM, VIC, JOE, HEX, CY3, NED, TAMRA, ROX, TEXAS RED, CY5 and the like. The invention further discloses a primer pair used for gene amplification; the primer pair comprises a forward primer and a reverse primer; the forward primer is an oligonucleotide which consists of base sequences of a sequence 3; the reverse primer is an oligonucleotide which consists of base sequences of a sequence 4. The invention further discloses a kit which contains the primers and the probe, and a detecting method for the CYP2C9*2 gene polymorphism.
Owner:FUWAI HOSPITAL OF CARDIOVASCULAR DESEASE CHINESE ACAD OF MEDICAL SCI
Test chipe of cytochrome P450 gene hereditary variation and its application
InactiveCN1912139AGuide rational drug useImprove throughputMicrobiological testing/measurementTherapeutic effectCYP2C9
The invention discloses a cytochrome P450 gene genetic variation detecting chip. It includes the probe fixed on the solid phase carrier to hybridize with the cytochrome P450 gene nucleotide sequence and / or complementation sequence. The invention also discloses the used chip detecting method. The detecting chip can effectively detect subtype genetic variation of CYP2C9, CYP2C19, CYP2D6, CYP3A5, forecast the therapeutic effectiveness of over 40% clinic common medicine to realize individualization medical treatment.
Owner:SHANGHAI BIOCHIP
Reagent kit and method for using molecular beacon probe for detecting human CYP2C9 gene polymorphism and application thereof
InactiveCN107653317AQuick checkEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationNucleotideNucleic acid sequencing
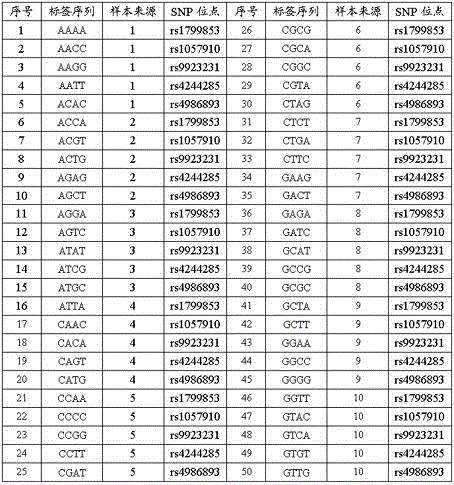
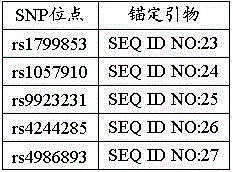
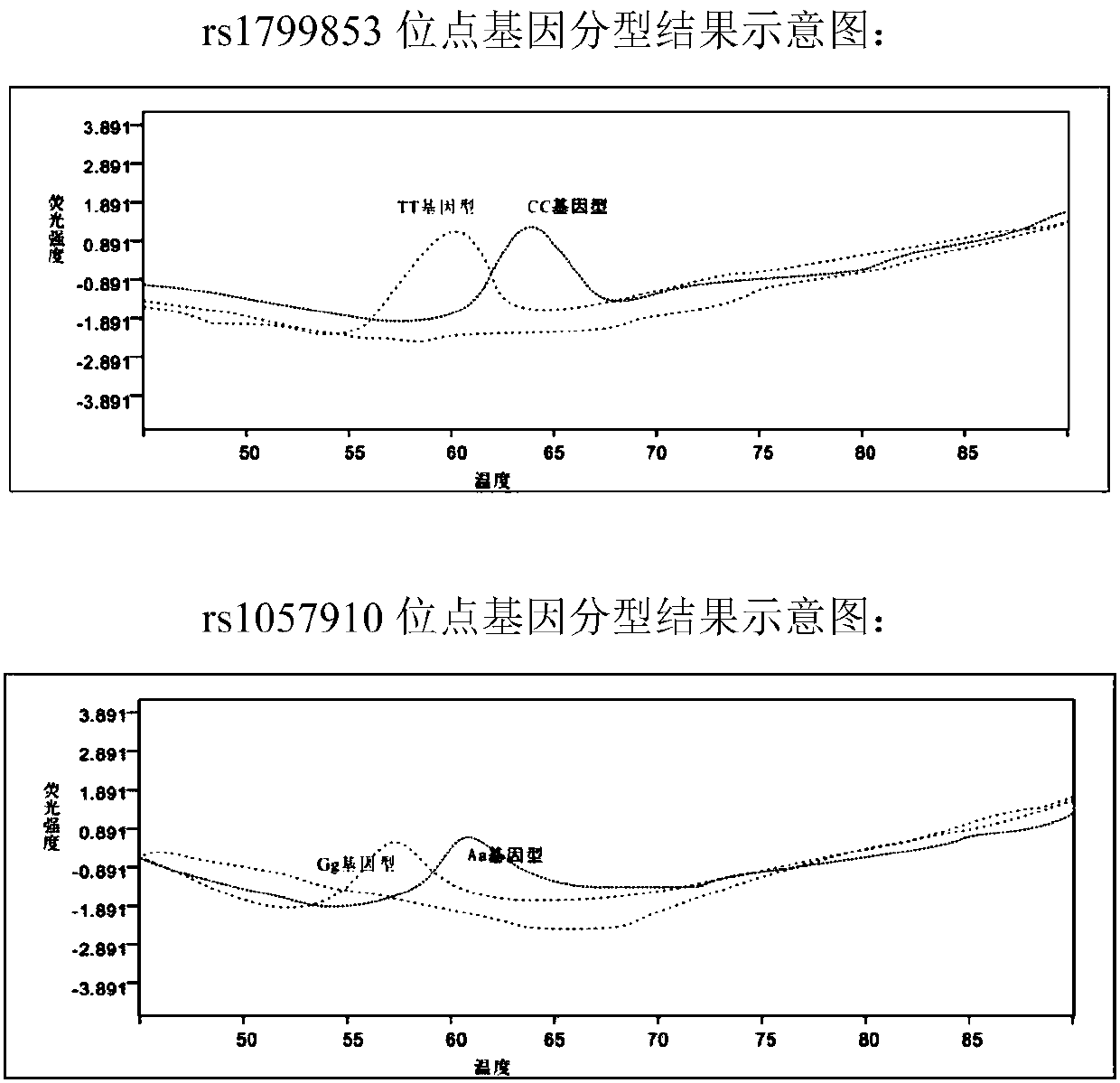
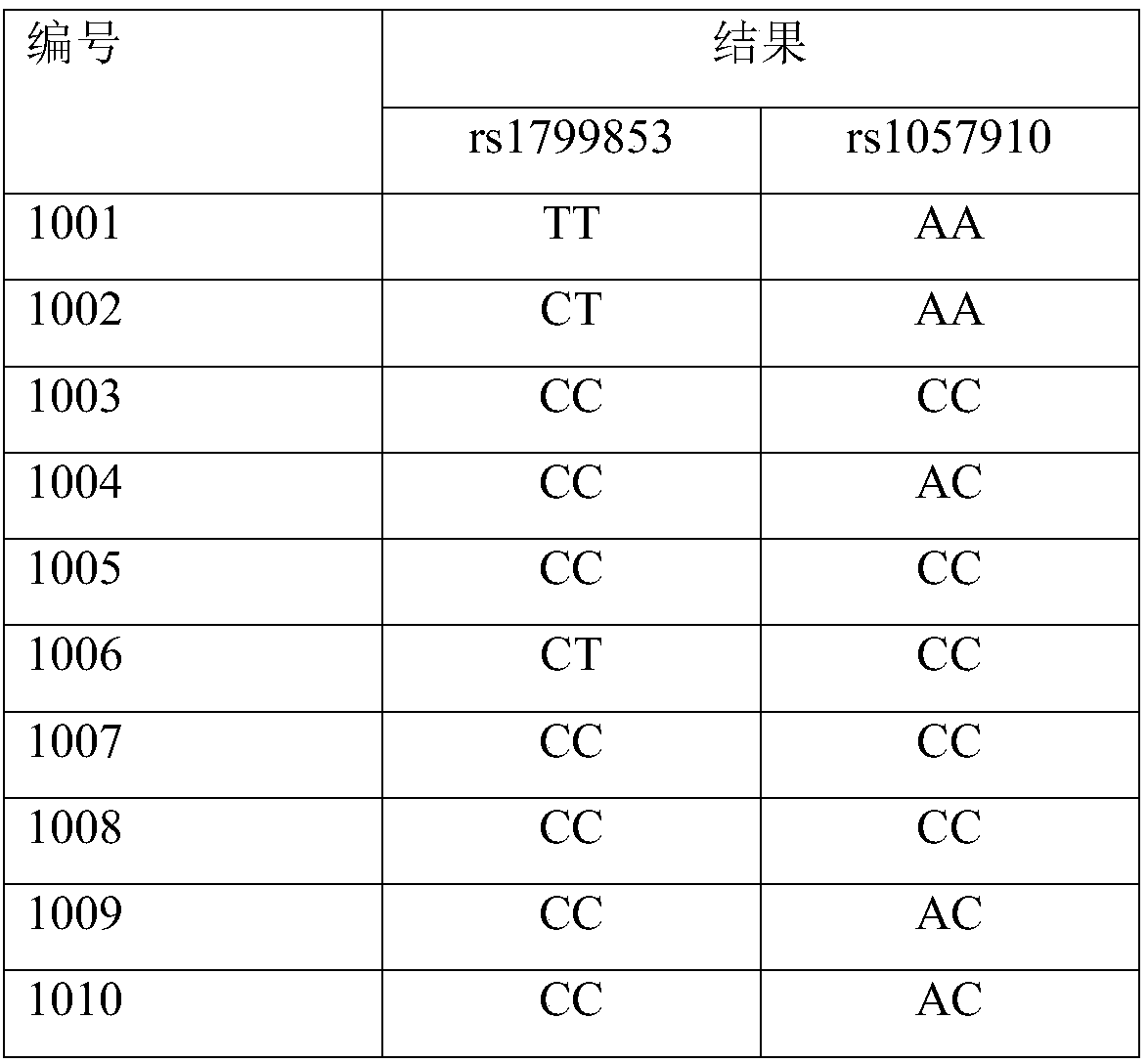
The invention provides a reagent kit for using a molecular beacon probe for detecting human CYP2C9 gene polymorphism. The reagent kit comprises the following nucleotide sequences, a primer pair and the probe for detection, wherein the primer pair is used for amplifying polymorphic sites of rs1799853 and rs1057910 of a CYP2C9 gene. The invention further discloses application on CYP2C9 gene polymorphic site detectionand amplification of the primer and the probe. The reagent kit for using the molecular beacon probe for detecting the human CYP2C9 gene polymorphism is easy to operate, precise in result and easy to interpret, polymorphism of the sites of rs1799853 and rs1057910 of the CYP2C9 gene can be detected rapidly in PCR. Compared with a sequencing method, a method for using the molecularbeacon probe for detecting the human CYP2C9 gene polymorphism does not need to conduct a series of follow-up treatment on PCR products, the PCR amplification and detection are carried out synchronously, and a cover does not need to be opened in the whole detection process, so that risks for contaminating the PCR products are reduced, the detection time is dramatically shortened, and the detectioncost is reduced.
Owner:黑龙江迪安医学检验所有限公司
Primer composition and kit for detecting sulfonylurea-related gene
InactiveCN108531579AQuick checkReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationSulfonylureaRefractory
The invention relates to a primer composition and a kit for detecting sulfonylurea-related genes, wherein the primer composition comprises a CYP2C9 (cytochrosome P4502C9) primer set, a KCNJ11 primer set and a ABCC8 primer set, and the kit comprises the above-mentioned primer compositions. Compared with the prior art, with ARMS (the amplification refractory mutation system) technology combined withSYBR dye, the kit for rapidly, sensitively and simply detecting the polymorphisms of sulfonylurea-related genes such as CYP2C9, KCNJ11 and ABCC8 is developed, and the kit comprises a specific ARMS detection primer, an internal control primer and aPCR reaction solution. By designing ARMS primers and replacing Scorpions probes with SYBR dyes, the primer composition and the kit for detecting the sulfonylurea-related genes have the advantages of greatly reducing detection costs, being suitable for for detection of CYP2C9, KCNJ11 and ABCC8 gene polymorphisms in Chinese patients, being rapid in detection, high in sensitivity, strong in specificity, simple in method and accurate in results, and being suitable for popularization and application.
Owner:宁波美丽人生医学检验所有限公司
CYP2C9 gene fragment containing 1300A>T mutation, protein fragment encoded through same and application thereof
ActiveCN103173443AMicrobiological testing/measurementOxidoreductasesBase JAllele-specific oligonucleotide
The invention belongs to the field of biology, relates to single-base mutation and particularly relates to a 1300th bit mutation site of a CYP2C9 gene corresponding to SEQ ID NO.2. The 1300th bit mutation site is mutated from wild type A into T. The invention relates to a nucleic acid fragment containing the 1300th bit mutation site, a protein fragment encoded through the same and application thereof. The invention also provides allelomorphic gene specific oligonucleotide for detecting the 1300th bit mutation site, a kit and a detection method.
Owner:BEIJING HOSPITAL
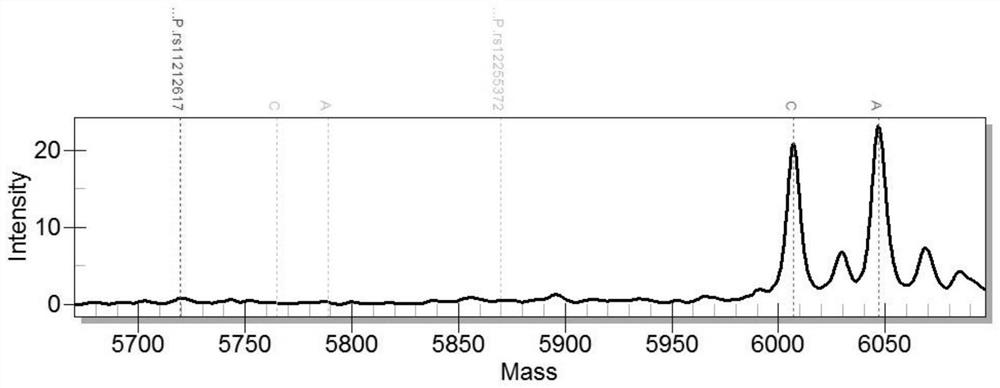
SNP site detection kit for guiding diabetes medication and use method thereof
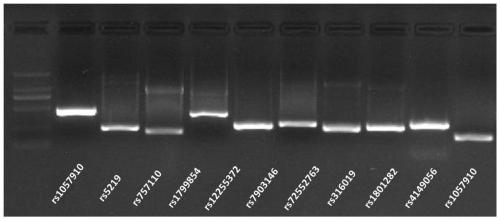
PendingCN109957617AEasy to operateReduce mistakesMicrobiological testing/measurementMeglitinideThiazolidinedione
The invention relates to a SNP site detection kit for guiding diabetes medication and use method thereof. The kit includes an amplification PCR reaction system, the amplification PCR reaction system comprises a PCR premix liquid and a plurality of pairs of PCR primers, through the multiple pairs of the PCR primers, 10 sites of 8 genes can be simultaneously detected, namely CYP2C9(rs1057910), KCNJ11(rs5219), ABCC8(rs757110, rs1799854), TCF7L2(rs12255372, rs7903146), SLC22A1(rs72552763), SLC22A2(rs316019), PPARG(rs1801282), and SLCO1B1(rs4149056), and the sequencing results guide the diabetes medication for patients. The 10 sites are detect and simultaneously amplified by adopting the method of multiplex PCR amplification, and the single reaction system amplification not only simplifies theoperation, reduces the possibility of errors, but also reduces the experiment cost. In addition, the guiding medication of the present invention cover four major classes of diabetes drugs: sulfonylureas, biguanides, thiazolidinediones and meglitinides, and the detection and analysis of 10 sites of related genes is more extensive than similar products on the market.
Owner:NANJING GEZHI GEMONICS CO LTD
Juno<TM>-based safety medication detection kit for children and chip
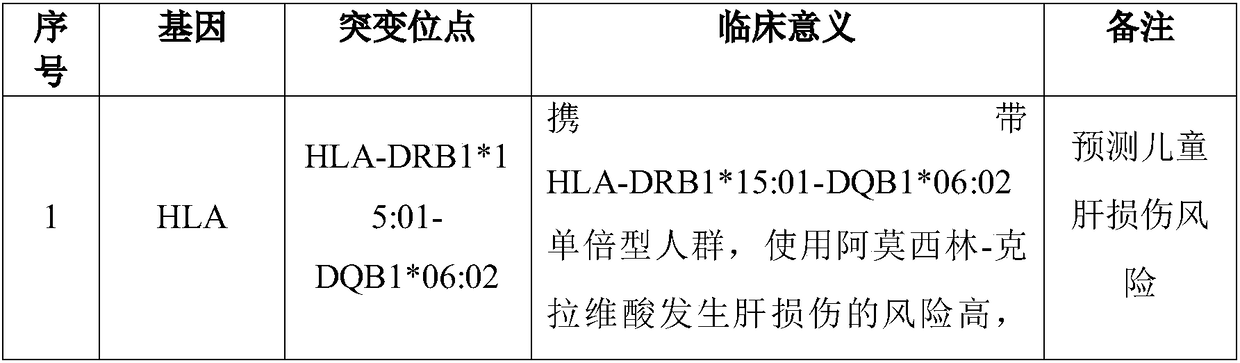
InactiveCN108728524AGood curative effectReduce the risk of adverse reactionsMicrobiological testing/measurementDNA/RNA fragmentationHLA-BCYP2C9
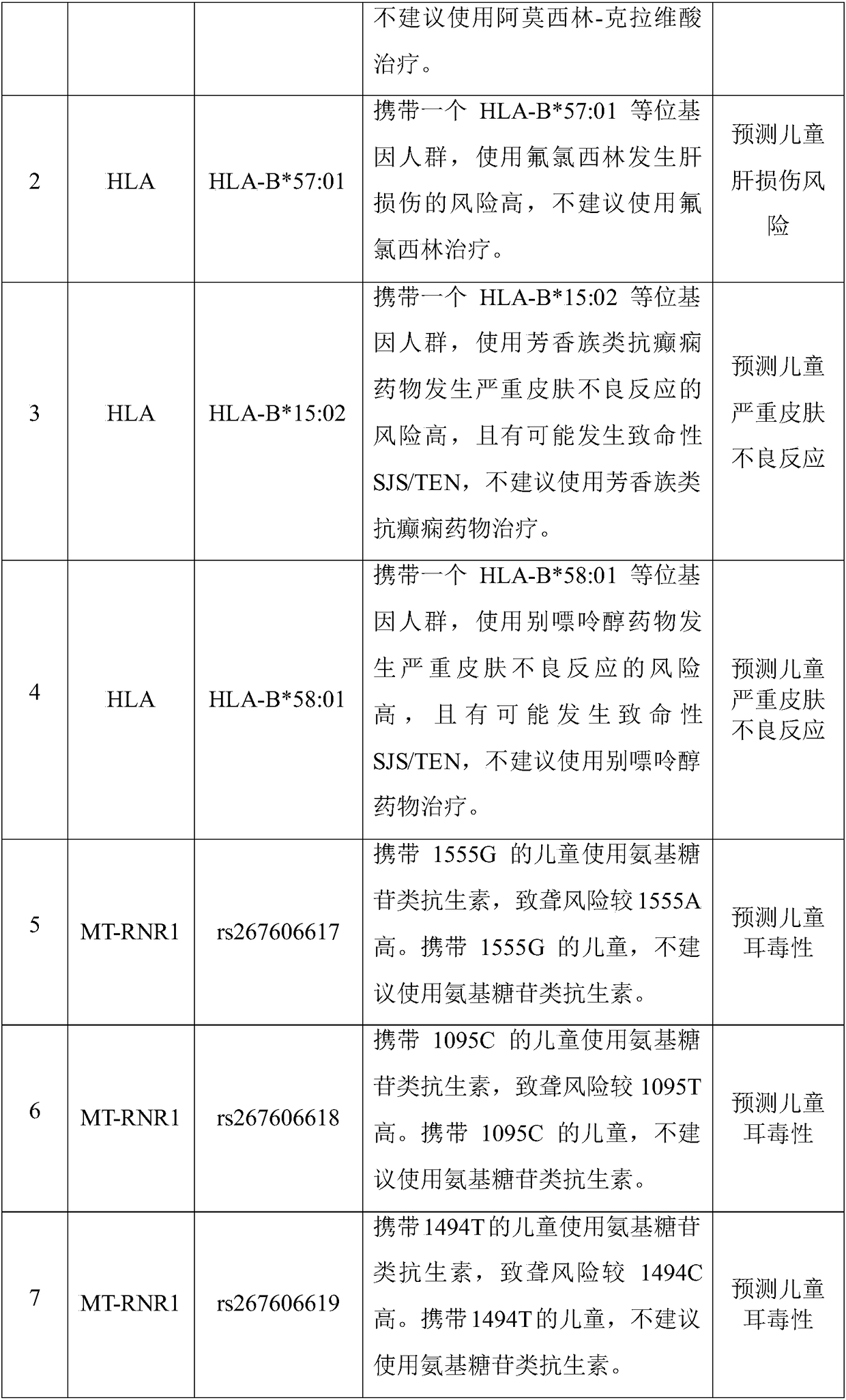
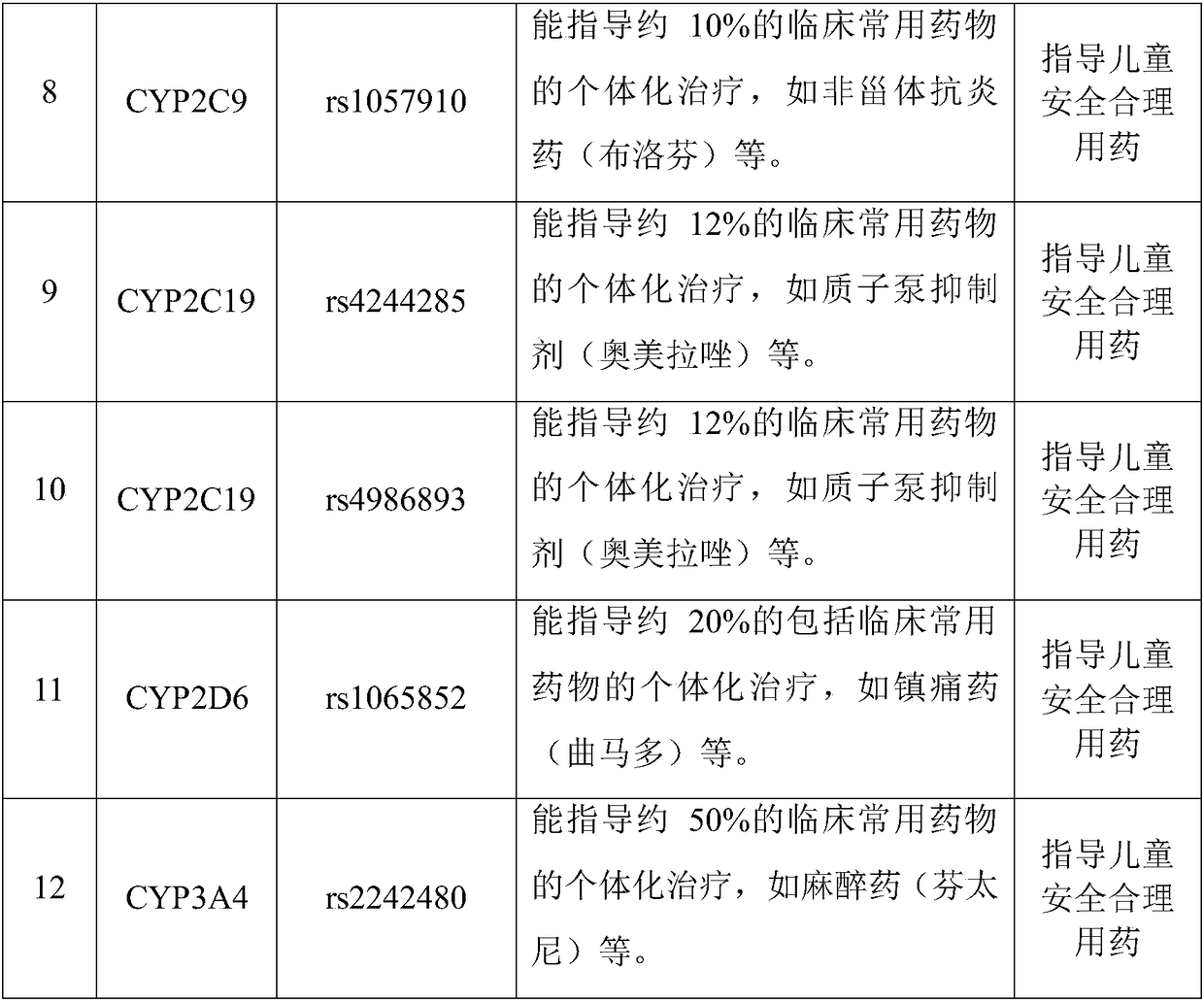
The invention discloses a Juno<TM>-based safety medication detection kit for children and a chip. The kit and the chip comprise primers at the following loci: HLA-DRB1*15:01-DQB1*06:02 loci of an HLAgene, an HLA-B*57:01 locus of the HLA gene, an HLA-B*15:02 locus of the HLA gene, an HLA-B*58:01 locus of the HLA gene, a rs267606617 locus of an MT-RNR1 gene,a rs267606618 locus of the MT-RNR1 gene,a rs267606619 locus of the MT-RNR1 gene, a rs1057910 locus of a CYP2C9 gene, a rs4244285 locus of a CYP2C19 gene, a rs4986893 locus of the CYP2C19 gene, a rs1065852 locus of a CYP2D6 gene, and a rs2242480 locus of a CYP3A4 gene.
Owner:THE THIRD XIANGYA HOSPITAL OF CENT SOUTH UNIV +1
Primer and method for detecting CYP2C9*2 gene polymorphism
InactiveCN104988145AStrong specificityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionMedicine
The invention provides a primer for detecting CYP2C9*2 gene polymorphism. The primer for detecting CYP2C9*2 gene polymorphism comprises a PCR amplification primer and a SNaPshot PCR primer. The primer for detecting CYP2C9*2 gene polymorphism belongs to the technical field of biological detection. The primer for detecting CYP2C9*2 gene polymorphism can achieve the specific detection on the CYP2C9*2 gene polymorphism, and the accuracy is good.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT +1
Primer group for gene group detection for medication guidance of anxiety patients, related application and corresponding kit
PendingCN113249460AAccurate detectionFit specific frequencyMicrobiological testing/measurementDNA/RNA fragmentationCYP3A5CYP2C9
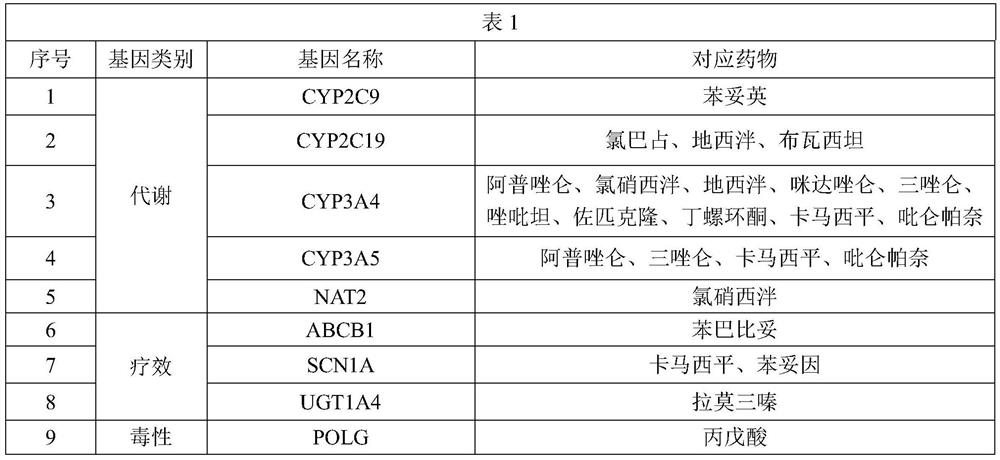
The invention provides a primer group for gene group detection for medication guidance of anxiety patients, related application and a corresponding kit, the primer group comprises primers for detecting variation related to each gene in the gene group, and the gene group comprises the following genes: CYP2C9, CYP2C19, CYP3A4, CYP3A5, NAT2, ABCB1, SCN1A, UGT1A4 and POLG.
Owner:上海恩元生物科技有限公司
Primer capable of detecting CYP2C9*2 gene mutation, and detection method
InactiveCN104673898AAvoid disadvantagesImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationNucleotideSample sequence
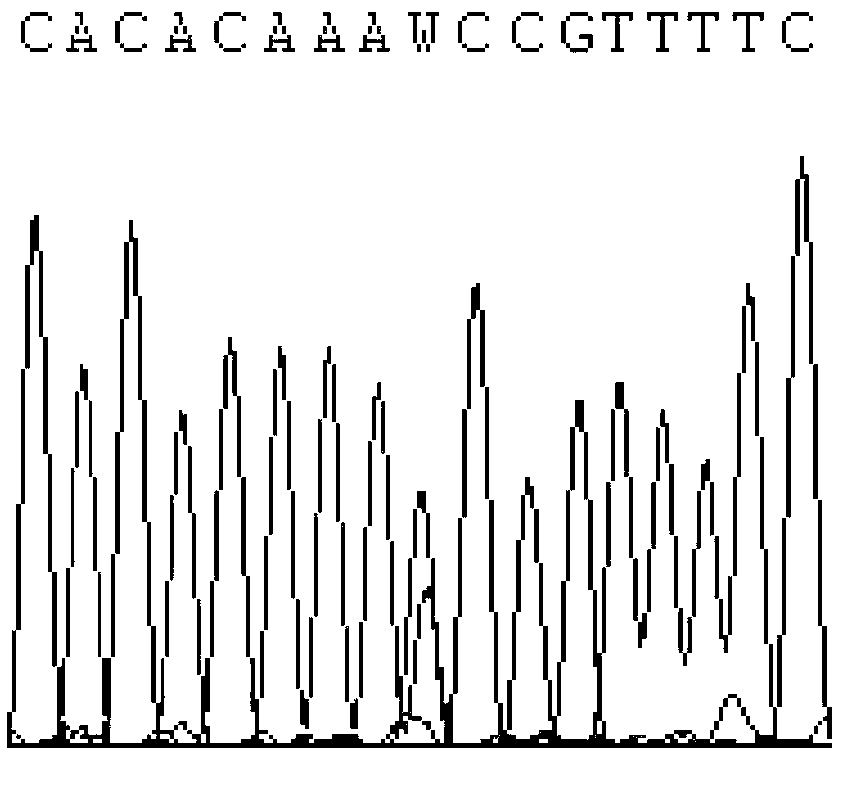
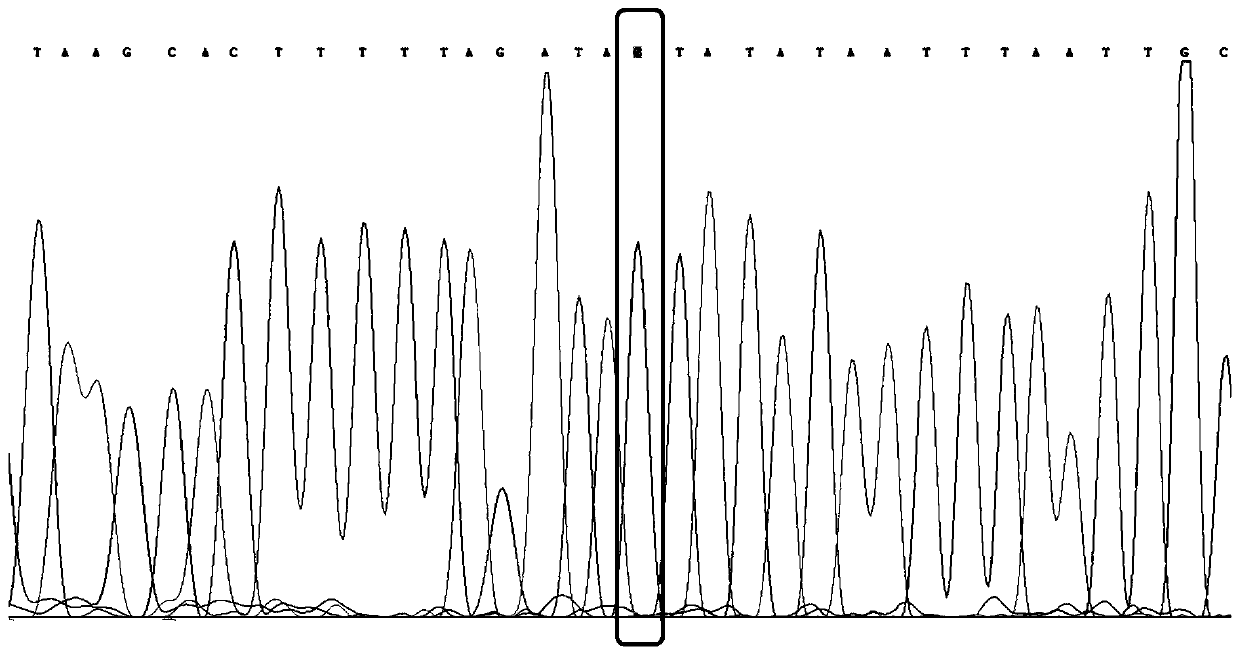
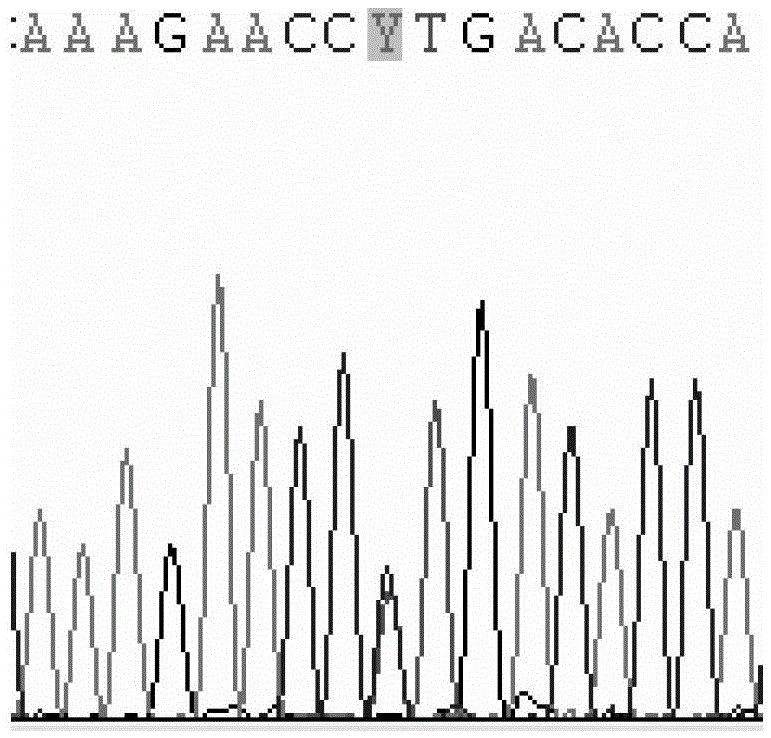
The invention discloses a primer capable of detecting CYP2C9*2 gene mutation, and a detection method. The invention provides a primer pair for CYP2C9*2 gene mutation, and the nucleotide sequences are shown in SEQ ID NO.1 and SEQ ID NO.2. The invention further discloses a method for detecting whether the CYP2C9*2 gene is mutated by applying the primer pair, and the method comprises the following steps: (1) extracting a to-be-detected blood sample DNA; (2) performing PCR amplification; (3) determining the PCR amplified segment sequence by adopting a Sanger method; and (4) comparing a CYP2C9*2 gene standard sequence and a sample sequence, judging detection locus base defined in the standard sequence and the corresponding locus base in the sequencing sequence, and analyzing the type of the gene mutation locus. The amplified segment is sequenced by adopting a Sanger method, and by comparing the sequencing result and the standard sequence, the mutation of the detected base can be intuitively read out, and the accuracy is high.
Owner:WUHAN ADICON CLINICAL LAB
Genetic typing detection primer group related to cardiovascular and cerebrovascular disease drugs, kit and detection method
PendingCN109402251AImprove featuresHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationCYP4F2 geneGenetic engineering
The invention relates to the field of genetic engineering and molecular biology, and provides a genetic typing detection primer group related to cardiovascular and cerebrovascular disease drugs, whichis used for specific amplification of nucleic acid fragments containing a site to be measured, the site to be measured comprises an rs1799853 site of a CYP2C9 gene, an rs1057910 site of the CYP2C9 gene, an rs2108622 site of a CYP4F2 gene and the like, the primer group comprises an rs1799853 primer group, an rs1057910 primer group, and an rs2108622 primer group and the like. The invention also provides a gene detection kit and a detection method. The gene detection primer group can carry out specific amplification on the nucleic acid fragments containing the sites, so that the sensitivity of gene mutation detection is high and the false positive rate is low. According to the invention, a second generation high-throughput gene sequencing technology is used to detect the genotypes of the sites to be measured, which makes the detection cycle short and the flux high.
Owner:WUHAN CONSIDERIN GENE & HEALTH TECH CO LTD
Detection of curative effect of hypoglycemic drug
InactiveCN101928761AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceCurative effect
The invention discloses a kit for detecting the curative effect of an individual hypoglycemic drug. The kit comprises a specific primer pair, a specific fluorescent probe pair, a fluorescence quantitative PCR conventional assembly and the like, and the specific primer pair is used for detecting a medication target site, namely a CYP2C9 gene and CYP2C19 gene polymorphism site of the hypoglycemic drug. The kit can be used for estimating the curative effect of the individual hypoglycemic drug by detecting the medication target site, namely the CYP2C9 gene and CYP2C19 gene polymorphism site of the hypoglycemic drug.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Primer set and kit for detecting polymorphism of hyperglycemia drug metabolism related genes and application of primer set and kit
PendingCN112210598AReduce adverse reactionsAccurate typingMicrobiological testing/measurementDNA/RNA fragmentationRelated geneMedication use
The invention relates to the technical field of medicine and biology, and discloses a primer set and a kit for detecting polymorphism of hyperglycemia drug metabolism related genes and application ofthe primer set and the kit. The primer set comprises primers capable of amplifying at least one hyperglycemia drug metabolism related gene: CYP2C9, KCNJ11, PPARgamma, SLCO1B1, SLC22A1, SLC22A2, and APOE. The primer set can also comprise a sequencing primer for carrying out Sanger sequencing on at least one of the above genes. The primer set and kit can formulate different schemes for hyperglycemiapatients according to individual differences of genes, select appropriate hypoglycemic drugs, realize accurate typing and precise medication, thereby increase hypoglycemic effect and reduce adverse drug reactions. The preferred primer set has high sensitivity and specificity, and when the preferred primer set is used for genotyping genes related to hyperglycemia drug metabolism, the primer set has advantages of being accurate in qualitative analysis, high in specificity and the like.
Owner:PRECEDO PHARMA CO LTD
Medicine metabolic relevant loci detection method
InactiveCN101760528BAccurate and reliable metabolic strengthAvoid adverse reactionsMicrobiological testing/measurementDrug metabolismFluorescence
The invention relates to a medicine metabolic relevant loci detection method, which comprises the following steps: extracting genome DNA from human samples; respectively designing a Taqman probe pair and a primer pair according to at least two medicine metabolic relevant genes; respectively marking the 5' end and the 3'end of the Taqman probe pair with fluorescence reporting genes and fluorescence quenching genes; carrying out fluorescence quantitative PCR augmentation on the genome DNA; and judging whether the medicine metabolic relevant genes have the mutation according to the fluorescence quantitative PCR augmentation results. Preferably, the number of the medicine metabolic relevant genes is four, the Taqman probe pair and the primer pair are used for detecting a loci rs1057910 of a gene CYP2C9, a loci rs4244285 of a gene CYP2C19, a loci rs4986893of a gene CYP2C19, a loci rs1065852 of a gene CYP2D6 and a loci rs28371759 of a gene CYP3A4. The invention has the advantages of ingenious design, simple operation and accurate and reliable detection results, and provides the reference frame for determining whether professional doctors are needed to be consulted so as to make sure themedicine can be taken or not or the proper dosage and the like when a certain medicine is taken.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
A near-infrared fluorescent probe for detecting cytochrome p450 2C9 and its application
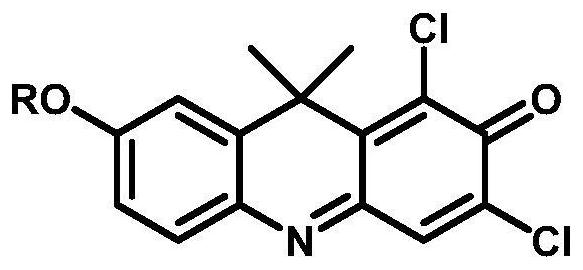
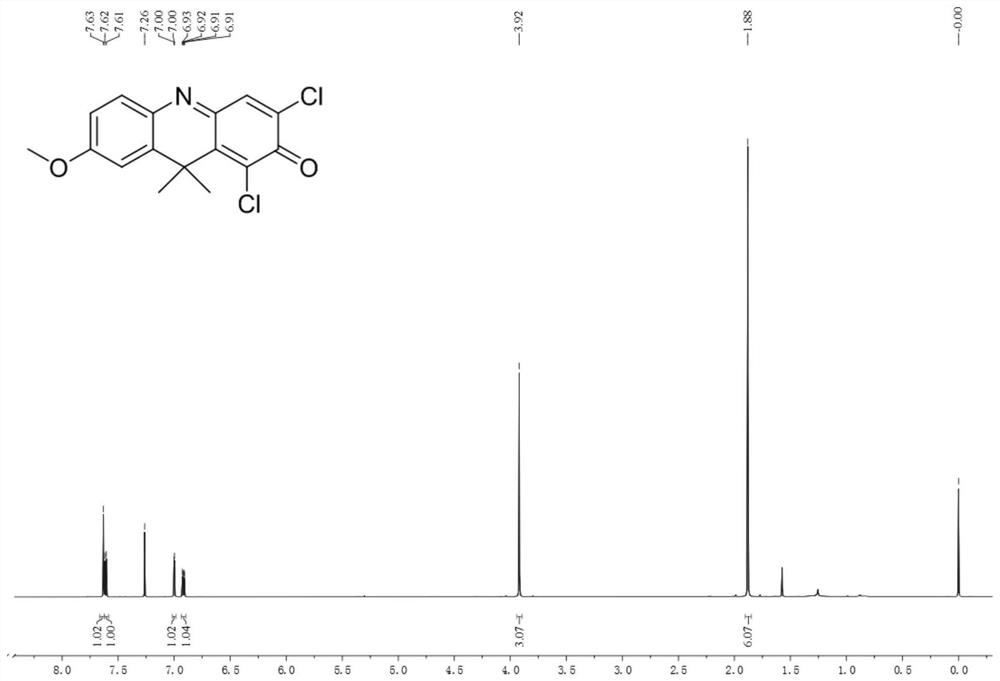
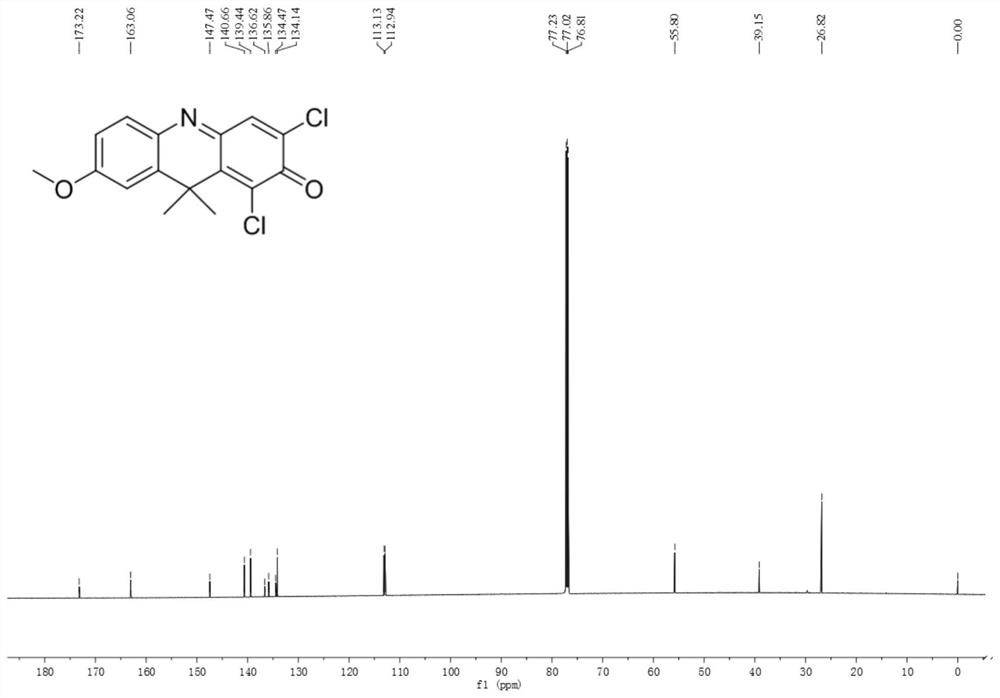
ActiveCN113720813BStrong specificityReduce testing costsOrganic chemistryMicrobiological testing/measurementFluoProbesDrug interaction
A near-infrared fluorescent probe for detecting cytochrome P450 2C9 and its application belong to the technical field of biomedicine. The specific probe substrate can be used to measure the enzymatic activity of CYP2C9 in biological systems. The process of CYP2C9 enzyme activity determination is as follows: 1,3-dichloro-7-alkoxy-9,9-dimethyl-2(9H)-acridone was selected O The dealkylation reaction is a probe reaction, which quantitatively detects the elimination amount of the substrate 1,3-dichloro-7-alkoxy-9,9-dimethyl-2(9H)-acridone in unit time The activity of CYP2C9 enzymes in various biological samples was determined by the amount of its dealkylated metabolites. The invention can be used for quantitative assessment of CYP2C9 enzyme activity in biological samples from different sources, species and individuals and imaging of CYP2C9 in organisms, so as to realize the assessment of CYP2C9's ability to dispose of drugs. It can also be used for rapid in vitro screening of CYP2C9 activity modulators, and to evaluate the potential of drug-drug interactions due to inhibition or activation of CYP2C9 catalytic activity, such as drugs and candidate compounds.
Owner:XUZHOU MEDICAL UNIV
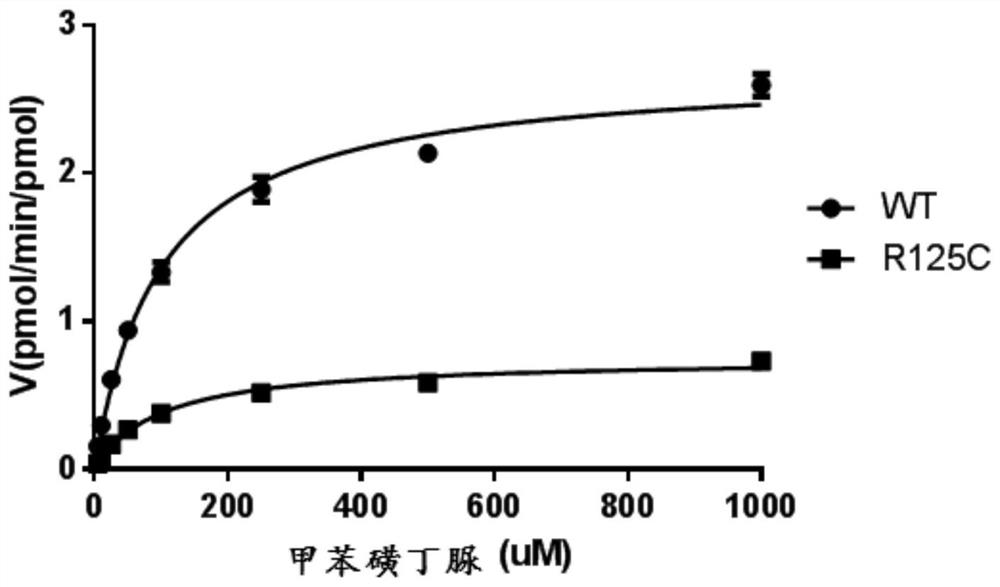
CYP2C9 gene segment containing 373C>T mutation, encoded protein segment and application of CYP2C9 gene segment
InactiveCN112458158AReduced metabolic activityMicrobiological testing/measurementDNA/RNA fragmentationBase JMutated protein
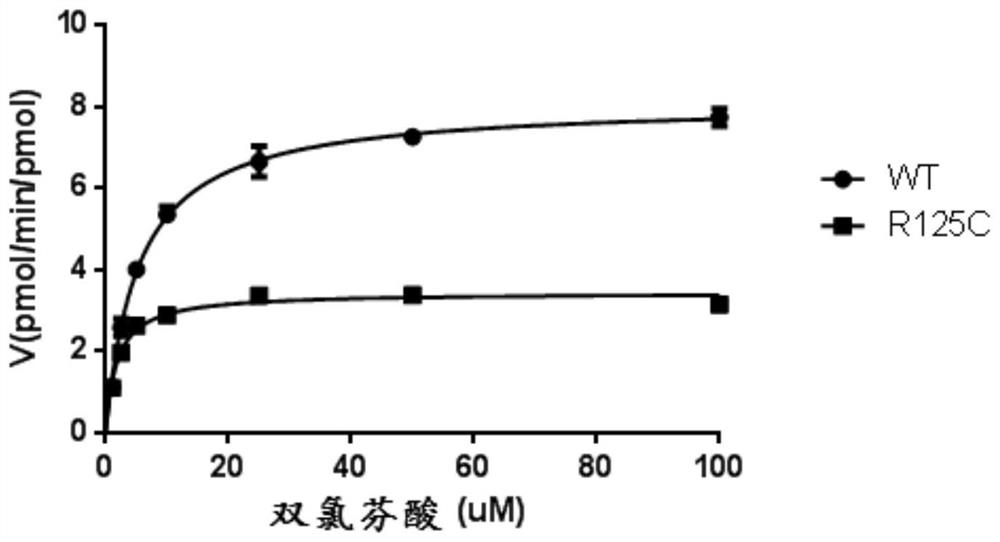
The invention discloses a CYP2C9 gene segment containing the 373C>T mutation, a coded protein segment and an application of the CYP2C9 gene segment, belongs to the field of biology, and relates to single base mutations. More specifically, the invention relates to a mutation site, corresponding to a 373th site of SEQ ID NO.2, of a CYP2C9 gene, a nucleic acid fragment containing the mutation site, aprotein fragment coded by the nucleic acid fragment, and an application of the nucleic acid fragment, wherein the site is mutated from wild type C to T. The mutant CYP2C9 protein, namely R125C, caused by the single base mutation has lower metabolic activity of drugs compared than wild type CYP2C9 protein, and therefore the guiding significance is provided for the medication of individuals carrying the mutation site.
Owner:杨杰孚 +1
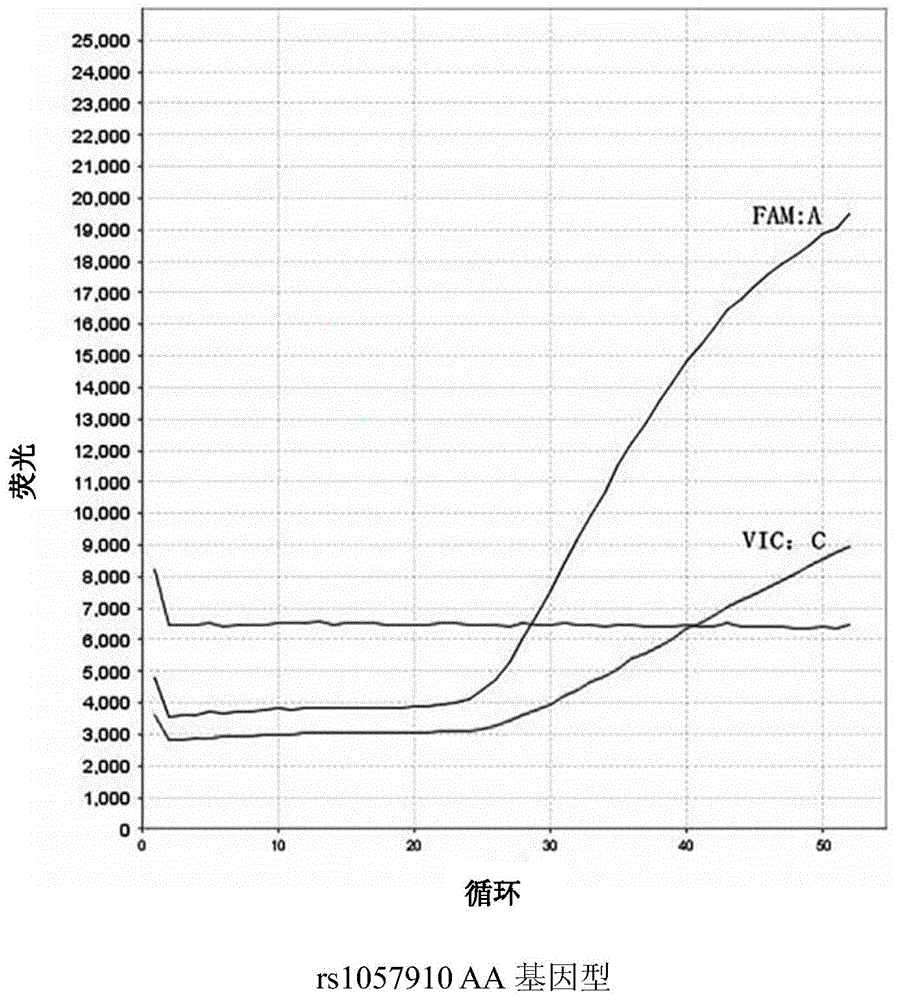
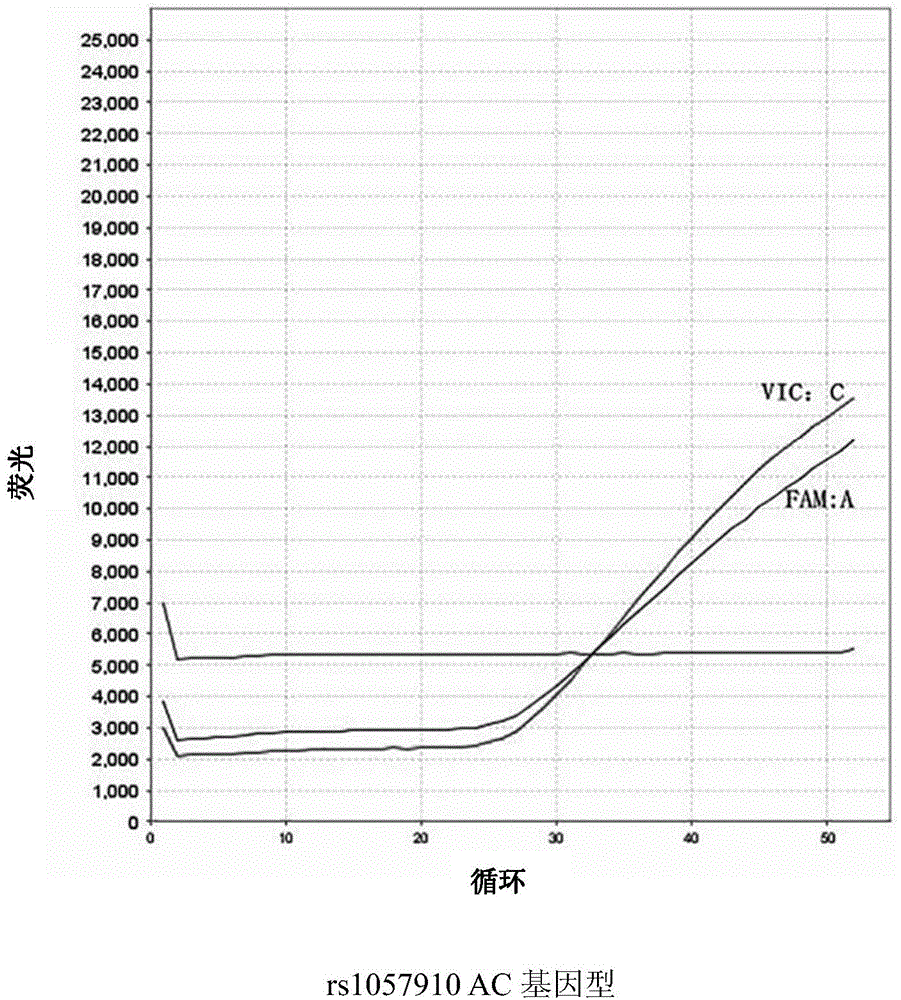
Kit and method for determining genotype of predetermined SNP (single nucleotide polymorphisms) locus of DNA (deoxyribonucleic acid) sample to be detected
InactiveCN104975069AEfficient determinationLow costMicrobiological testing/measurementFluorescenceNucleotide
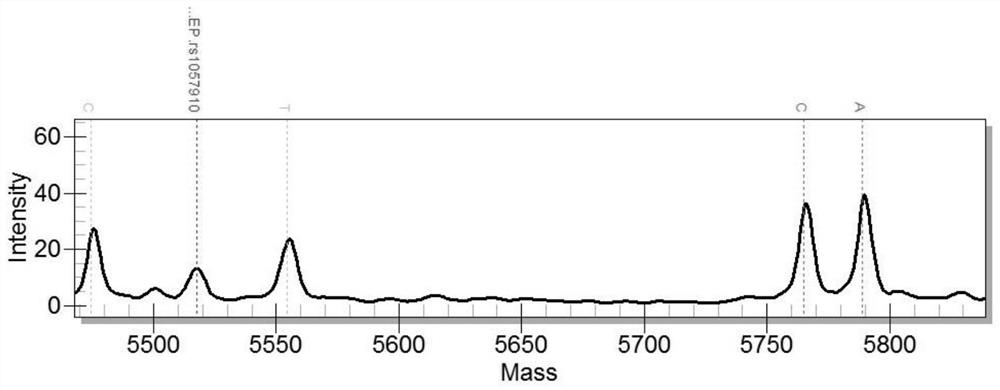
The invention discloses a kit and a method for determining the genotype of a predetermined SNP (single nucleotide polymorphisms) locus of a DNA (deoxyribonucleic acid) sample to be detected. The kit comprises a first nucleic acid molecule, a second nucleic acid molecule, a first probe and a second probe, wherein the 5' terminals of the probes are labelled with fluorescence reporter groups and the 3' terminals are labelled with fluorescence quenching groups; the fluorescence reporter groups of the first and second probes are different. The kit can be used for detection and genotyping of an rs1057910 locus of a CYP2C9 gene.
Owner:湖北维达健基因技术有限公司
Reagents and Methods for Detecting CYP2C9 Polymorphisms
The present invention relates to oligonucleotide sequences for amplification primers and detection probes and their use in nucleic acid amplification methods for the specific detection of clinically relevant CYP2C9 polymorphisms, in particular CYP2C9 polymorphisms associated with adverse drug response. The oligonucleotide sequences are also provided assembled as kits that can be used to predict how an individual will respond to drugs or other xenobiotic compounds that are metabolized, at least in part, by CYP2C9.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Specific primer and liquid phase chip for SNP (Single Nucleotide Polymorphism) detection of CYP2C9 (Cytochrome P4502C9)
ActiveCN102154455BImprove signal-to-noise ratioImplement parallel detectionMicrobiological testing/measurementDNA/RNA fragmentationMicrosphereGenetics
The invention discloses a specific primer and liquid phase chip for SNP (Single Nucleotide Polymorphism) detection of CYP2C9 (Cytochrome P4502C9), wherein the liquid phase chip comprises an ASPE primer consisting of a tag sequence of 5' end and a specific primer of 3' end aiming at target gene mutation, a microsphere coated by the anti-tag sequence, and an amplification primer; the specific primer includes: SEQ ID NO.17 and SEQ ID NO.18 aiming at G98A SNP locus, SEQ ID NO.19 and SEQ ID NO.20 aiming at G173A SNP locus, SEQ ID NO.21 and SEQ ID NO.22 aiming at C90T SNP locus, SEQ ID NO.23 and SEQ ID NO.24 aiming at A162C SNP locus, SEQ ID NO.25 and SEQ ID NO.26 aiming at C72T SNP locus, SEQ ID NO.27 and SEQ ID NO.28 aiming at T102C SNP locus, SEQ ID NO.29 and SEQ ID NO.30 aiming at A77G SNP locus, and / or SEQ ID NO.31 and SEQ ID NO.32 aiming at A167C SNP locus. The matching rate between a detection result obtained by adopting the liquid phase chip for SNP detection of CYP2C9 and a sequencing method can be 100%.
Owner:SUREXAM BIO TECH
The cyp2c9 gene fragment including 1400t>c mutation, the encoded protein fragment and its application
ActiveCN103194464BMicrobiological testing/measurementOxidoreductasesBase JAllele-specific oligonucleotide
Owner:BEIJING HOSPITAL
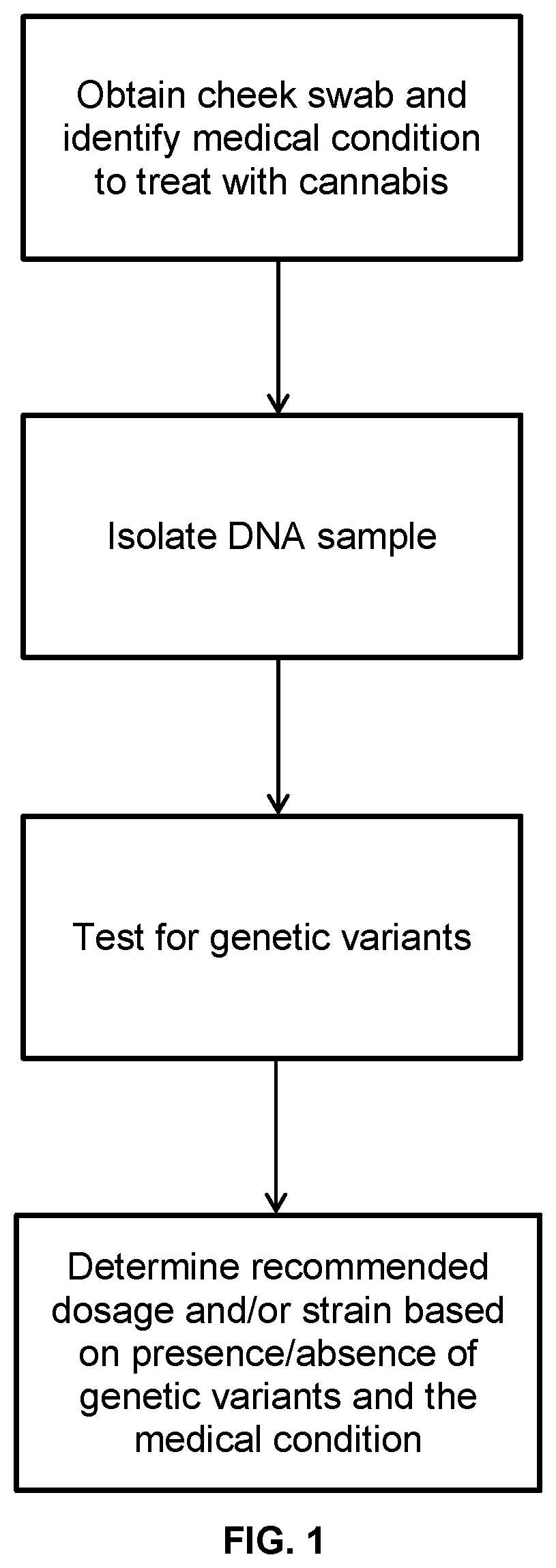
Dosage and varietal recommendations for the treatment of medical conditions using cannabis
InactiveUS20210172016A1Impeding determinationImprove patientHydroxy compound active ingredientsAntipyreticDiseaseCannabinoid
Described are methods for determining a recommended dosage and / or variety of cannabis for a subject based on genetic testing. The presence or absence of genetic variants in a sample from the subject is determined and used to determine a recommended dosage of cannabis, estimate the sensitivity of the subject to cannabis, or select a subject for the treatment of a medical condition. In some embodiments the genetic variants include polymorphisms in or near CYP2C9, CYP3A4 and / or CYP2C19, optionally that are associated with cannabinoid metabolism. The recommended dosage may be for a specific variety of cannabis for treating a medical condition.
Owner:AURORA CANNABIS ENTERPRISES INC
Detection of curative effect of anti-angina pectoris drug
InactiveCN101928760AMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceAngina
The invention discloses a kit for detecting the curative effect of an individual anti-angina pectoris drug. The kit comprises a specific primer pair and a specific fluorescent probe pair for detecting the polymorphic sites of an ALDH2 gene, a CYP3A4 gene, a CYP3A5 gene, a CYP2D6 gene and a CYP2C9 gene at the targeted use sites of the anti-angina pectoris drug, fluorescent quantitative PCR conventional components, etc. The kit estimates the curative effect of the individual anti-angina pectoris drug by detecting the polymorphic sites of the ALDH2 gene, the CYP3A4 gene, the CYP3A5 gene, the CYP2D6 gene and the CYP2C9 gene at the targeted use sites of the anti-angina pectoris drug.
Owner:XINBAXIANG SHANGHAI MOLECULAR MEDICAL TECH SHANGHAI
Kit for detecting human diabetes sensitive genes
InactiveCN112251509AAccurate detectionEasy to detectMicrobiological testing/measurementDiabetes mellitusMedicine
The present invention discloses a kit for detecting human diabetes sensitive genes. The kit comprises a C11orf 65 primer group, a CYP2C9 primer group, a TCF7L2 primer group, an ABCC8 primer group anda KCNJ11 primer group. The human diabetes drug sensitive genes C11orf 65, CYP2C9, TCF7L2, ABCC8 and KCNJ11 are used as detection objects, and variations of the related genes can be rapidly, simply andaccurately detected by combining the specific primers and combining a nucleic acid mass spectrum detection technology.
Owner:为康(苏州)基因科技有限公司
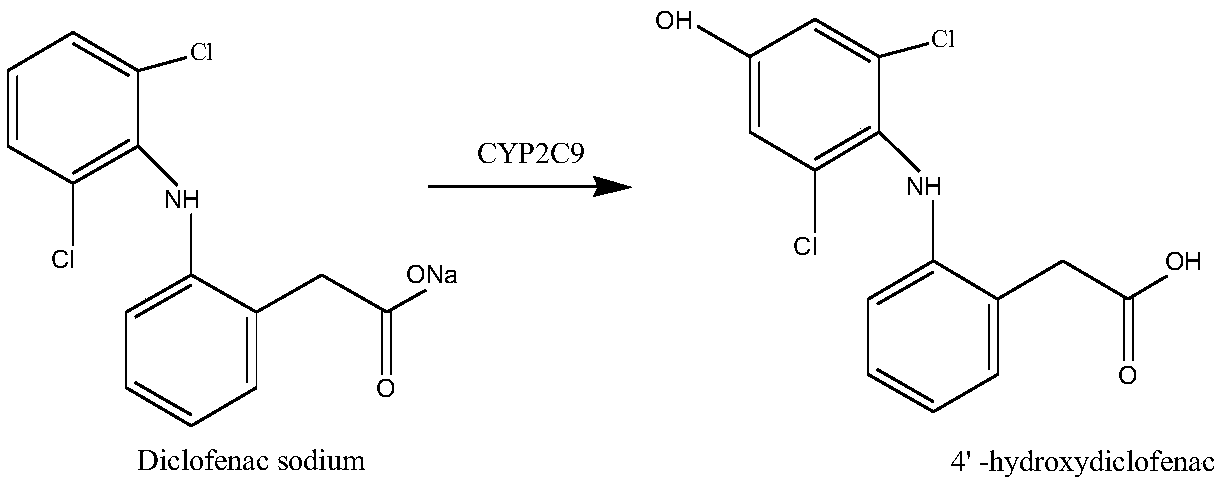
A method for detection of CYP2C9 enzyme activity in earthworms by high performance liquid chromatography tandem mass spectrometry
ActiveCN106290661BImprove accuracyHigh precisionComponent separationProtein mass spectrometryPollution
The invention relates to a method for detecting CYP2C9 enzyme activity in earthworms by adopting a high-performance liquid chromatography-tandem mass spectrometry and belongs to the technical field of enzyme activity detection. The method specifically comprises the following steps: (1) preparing earthworm microsome protein suspension; (2) adding the earthworm microsome protein suspension prepared in the step (1) into an incubation system containing a probe substrate; raising the temperature to start an enzymatic reaction; after the enzymatic reaction is stopped, detecting a metabolic product, which is generated after the enzymatic reaction is stopped, by adopting the high-performance liquid chromatography-tandem mass spectrometry; calculating the CYP2C9 enzyme activity. The detection method is high in accuracy, precision and sensitivity and high in stability, and can be used for analyzing and detecting the CYP2C9 enzyme activity in the earthworms under different pollution environments, so that a detection method for probing the possibility of taking CYP2C9 as a biological marker to indicate soil pollution is provided.
Owner:CHONGQING ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com