CYP2C9 gene segment containing 373C>T mutation, encoded protein segment and application of CYP2C9 gene segment
A technology of nucleic acid fragments and mutation sites, applied in the field of biology, can solve problems such as differences in drug efficacy, insufficiency, drug toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Identification of new mutation sites in human CYP2C9 gene
[0041] In this example, the blood samples of patients with clinically low warfarin dosage were collected, the genomic DNA in the blood was extracted, and sequencing primers were designed to amplify and sequence the 9 exons of the CYP2C9 gene to analyze whether the CYP2C9 gene There are mutation sites.
[0042] 1) DNA extraction:
[0043] Take 5ml of venous EDTA anticoagulated blood sample from the subject; then extract the genomic DNA of the blood sample to be tested according to the common salting-out method and / or using a special DNA extraction kit (purchased from Omega, USA).
[0044] 2) PCR amplification:
[0045] Amplification primers were designed to amplify the 9 exon sequences of the CYP2C9 gene in the obtained genomic DNA sample. The sequences of the amplification primer pairs are shown in Table 1.
[0046] Use 50μl PCR reaction system, including: 1×PCR buffer, 1.5mM MgCl 2 , 100-150ng ...
Embodiment 2
[0060] Embodiment 2: the expression of target gene
[0061] The open reading frame (ORF) of the R125C mutant of the present invention was respectively obtained by site-directed mutagenesis using the plasmid vector (gifted by Professor Zhou Shufeng from the University of South Florida) connected with the open reading frame of wild-type CYP2C9*1 as a template. The technique of site-directed mutagenesis is well known in the art, and those skilled in the art can undoubtedly know how to complete this step based on the determined template and target.
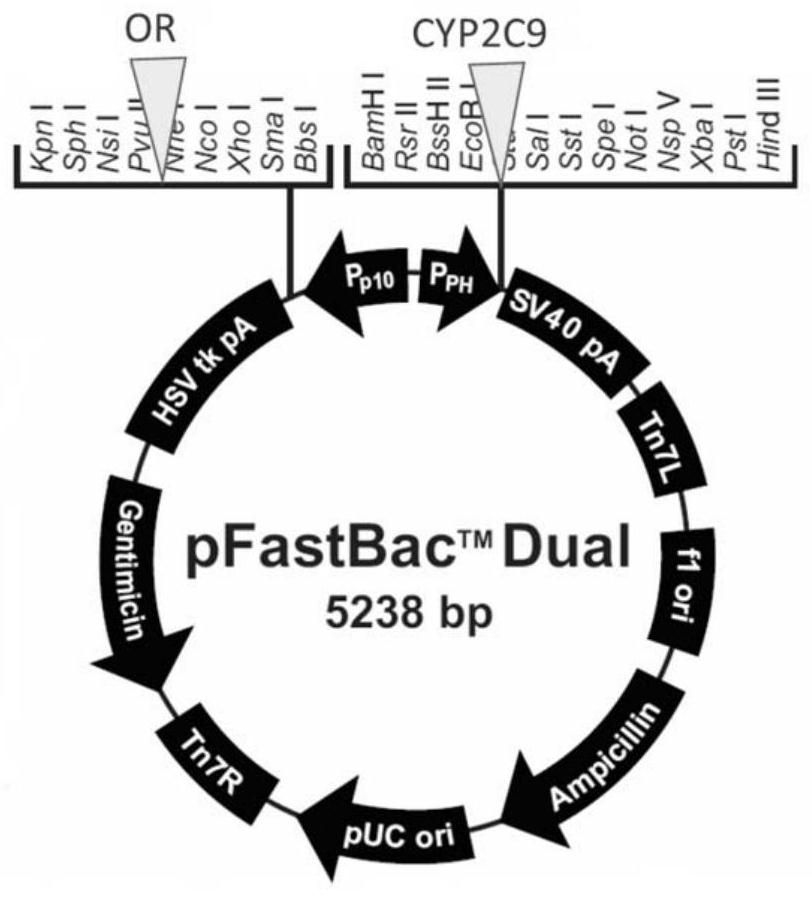
[0062] Then clone the ORF of the CYP2C9*1 gene and the mutant gene for site-directed mutagenesis into the vector pFastBac-dual connected with cytochrome P450 oxidoreductase (OR), so that the CYP2C9 gene and OR are respectively placed behind the PH and p10 promoters , to construct a dual expression vector expressing OR and CYP2C9 (or its mutants) simultaneously. The structure of pFastBac-dual vector and the insertion site of CYP2C9 ge...
Embodiment 3
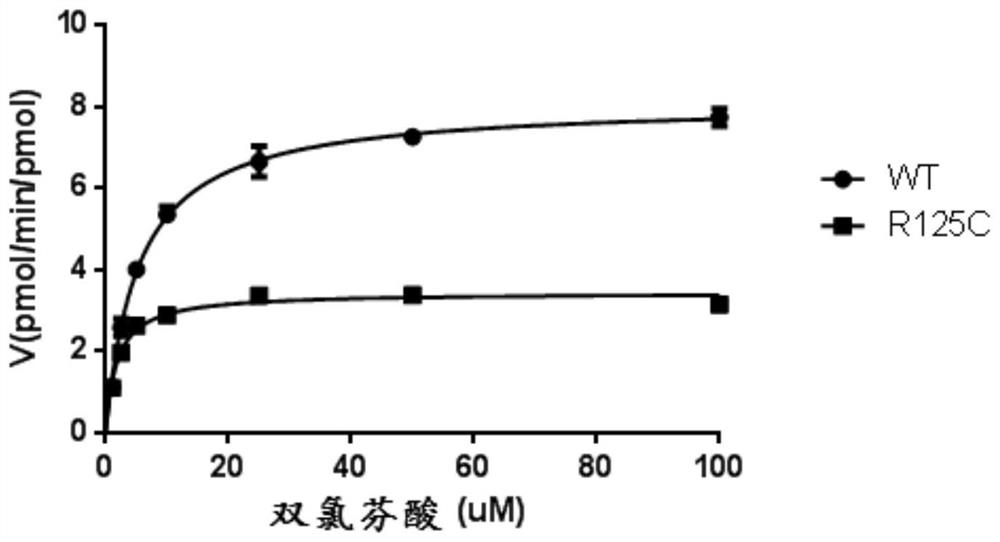
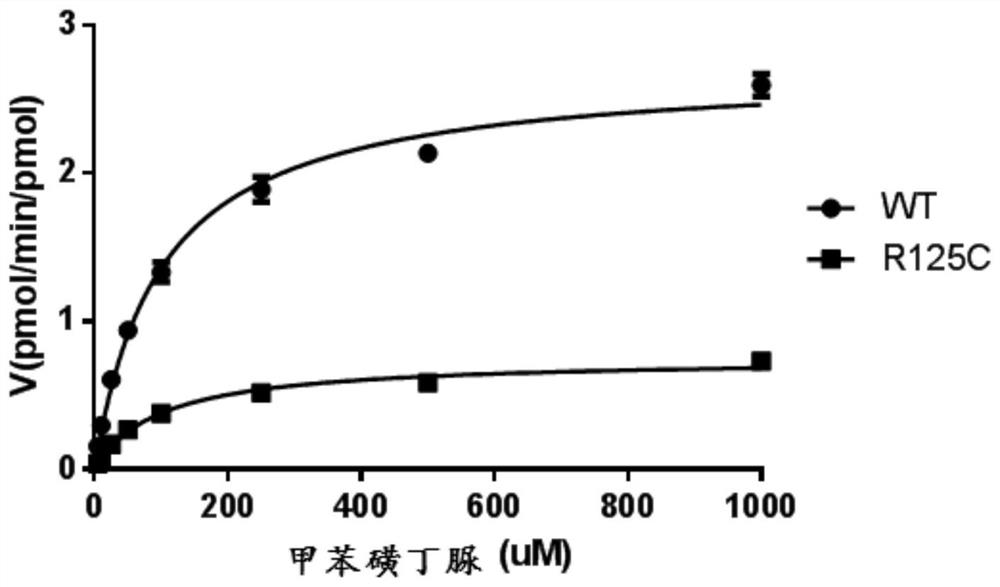
[0066] Example 3: In vitro analysis of the metabolic properties of diclofenac using the obtained insect microsomes:
[0067] 1) Chromatographic conditions: CORTECST UPLC C18 column (1.6μm, 2.1×50mm); phase A acetonitrile, phase B 0.1% formic acid-water, flow rate 0.4ml / min; 0-0.5min, 20%A; 0.5-1min , 20%-95%A; 1-2min, 95%A; 2-2.3min, 95%-20%A, running time 3min. The column temperature is 40°C.
[0068] 2) Mass spectrometry detection conditions:
[0069] The ion pair of 4-hydroxydiclofenac is 311.94>265.95, the cone voltage is 20v, and the collision energy is 18v; the internal standard diazepam ion pair is 285.1>193.1, the cone voltage is 35v, and the collision energy is 30v. Use the MRM model. The detection wavelength is: 280nm.
[0070] 3) Incubation conditions:
[0071]The total volume of the reaction was 200 μL, including: 100 mM Tris-HCl (pH 7.4), 1×NADPH coenzyme generation system (Promega, USA), 2 pmol cytochrome b5 and diclofenac (purchased from Sigma, USA, the fin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com