Wt1-origin HLA-DR-binding antigen peptide
A technology for HLA-DRB1 and cancer antigens, applied in the field of HLA-DRB1*0405-binding antigen peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] 1. Preparation of Dendritic Cells
[0193] Extraction of HLA-DRB1 from HLA-DRB1 by density gradient centrifugation using Ficoll-Paque * 0405-positive peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy volunteers. Divide the resulting 8 x 10 6 PBMCs were suspended in 2ml X-VIVO 15 containing 1% AB serum TM culture medium (Camblex), inoculated in a 6-well culture plate, and cultured for 2 hours. After culturing, non-adherent cells were removed and adherent cells were washed with Hanks solution. In X-VIVO 15 containing 1% AB serum, 1000U / ml IL-4 and 1000U / ml GM-CSF TM Culture adherent cells in culture medium. On days 2 and 4 of culture, half of the medium was replaced with fresh medium. On day 6, TNF-α was added to a final concentration of 100 U / ml. Cells that survived on day 7 were used as experimental dendritic cells.
[0194] 2. Preparation of CD4 positive T cells (helper T cells)
[0195] Blood obtained from the same healthy vol...
Embodiment 2
[0204] Establishment of CD4-positive T cell lines specific for WT1 peptide
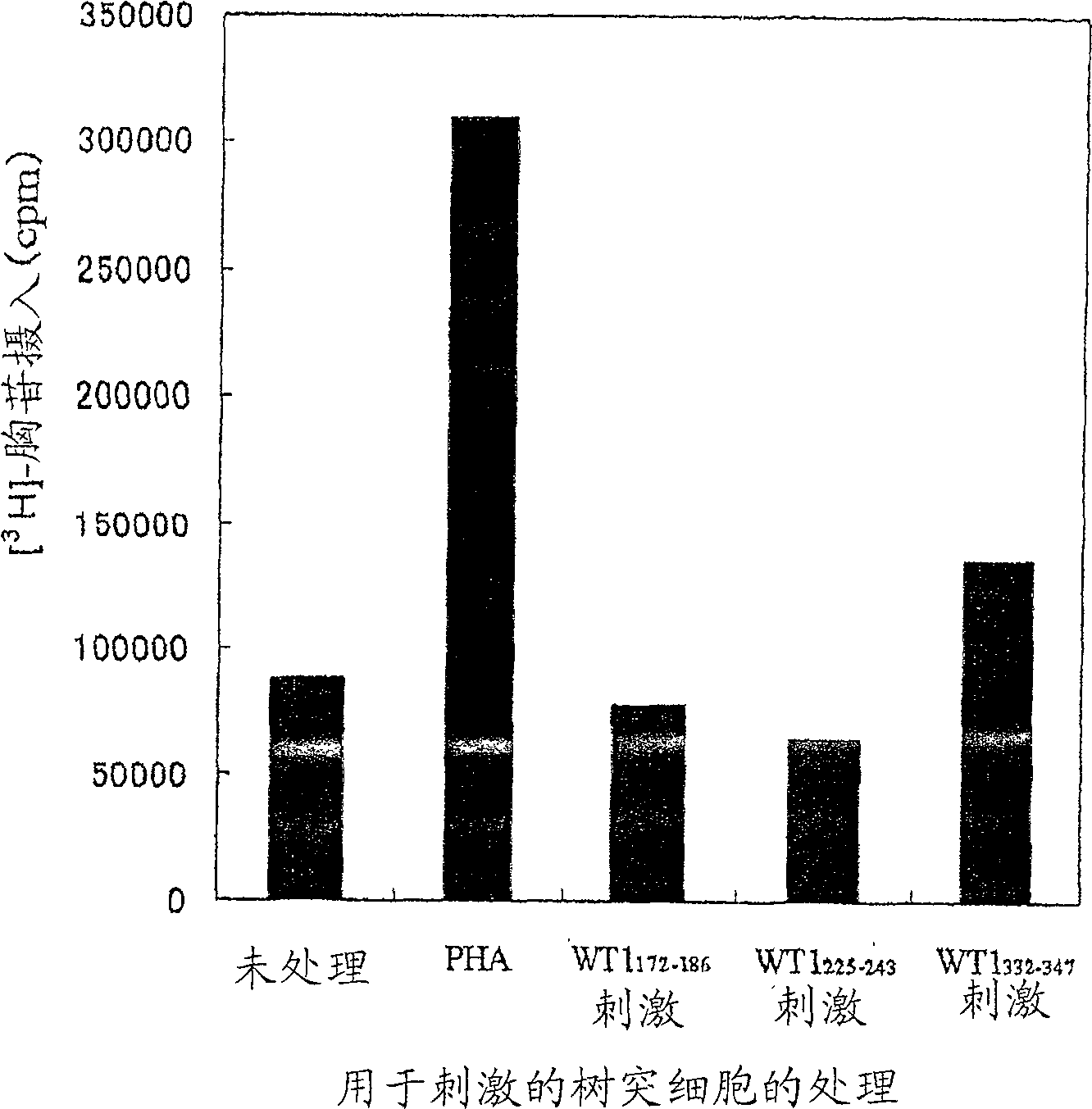
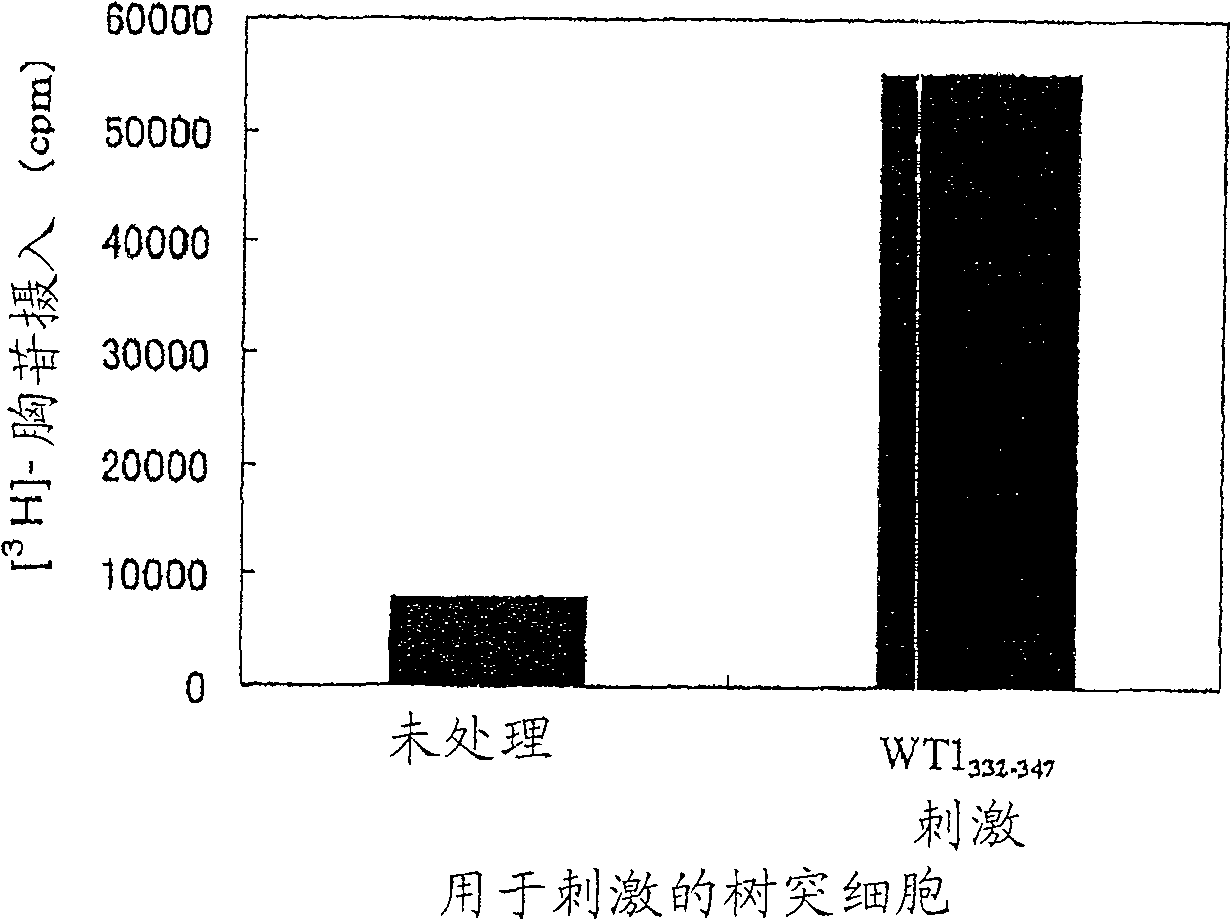
[0205] Dendritic cells prepared by a method similar to Example 1 were prepared in 10 4 Cells / well were seeded in 96-well plates, and then 10 3 Cells / well seeded as WT1 332-347 CD4 positive T cells induced by peptide (SEQ ID NO: 24). As for the medium, X-VIVO 15 containing 1% AB serum, 20 U / ml IL-2 and 5 μg / ml PHA was used TM Medium. The CD-4 positive T cell line was established by continuous culture and named "G2 cell line". The response of the G2 cell line to dendritic cells stimulated with the peptide was determined by a method similar to that of claim 1 . The results are shown in figure 2 middle. When used with WT1 332-347 The G2 cell line showed a proliferative response when co-cultured with peptide-stimulated dendritic cells but not when co-cultured with non-peptide-stimulated dendritic cells.
[0206] These results suggest that the G2 cell line is a WT1-specific 332-347 Peptide CD4 ...
Embodiment 3
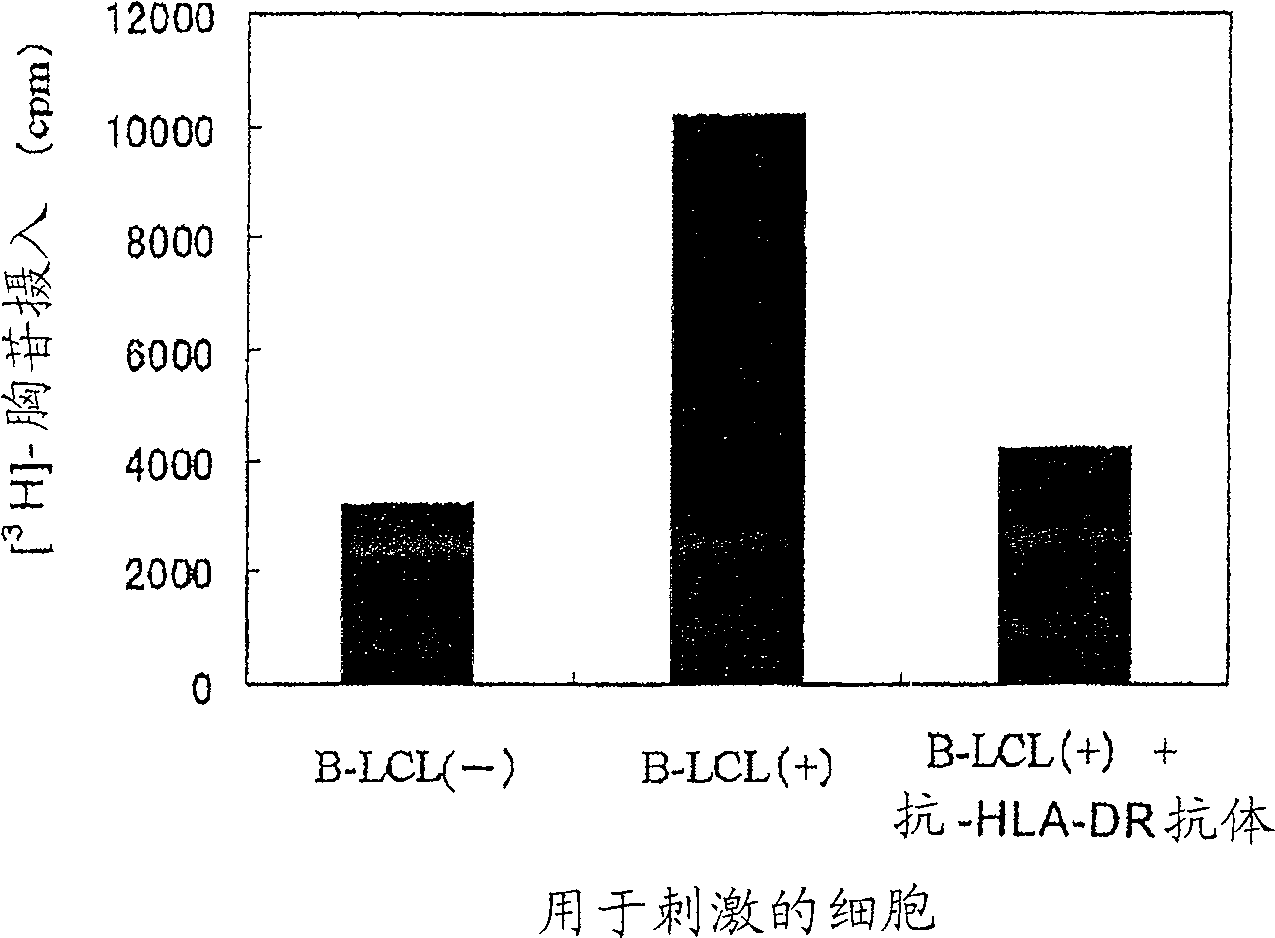
[0208] WT1 peptide antigen presentation to HLA-DR molecules
[0209] In a manner similar to Example 1, HLA-DRB1 was extracted from HLA-DRB1 by density gradient centrifugation using Ficoll-Paque * 0405-positive peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy volunteers. Then, with 10 7 Cells / well PBMCs were seeded in 24-well plates. As the medium, RPMI1640 medium containing 10% FCS and 55 µM 2ME was used. After addition of a medium containing Epstein-Barr virus (EBV), culture was continued for another 4 weeks to establish a B-cell line transformed with EBV, which was named "B-LCL(-) cells". EBV was prepared from the culture supernatant of B95-8 (JCRBCell Bank No. 9123), which is an EBV-producing cell line. Adjust B-LCL(-) cells to 3 x 10 7 cells / mL, and a medium containing a virus expressing the WT1 gene was added thereto, followed by addition of polypropylene (final concentration, 8 μg / mL), and the mixture was added to a 24-well plate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com