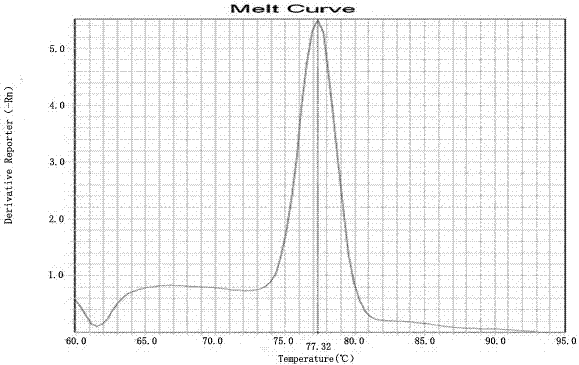
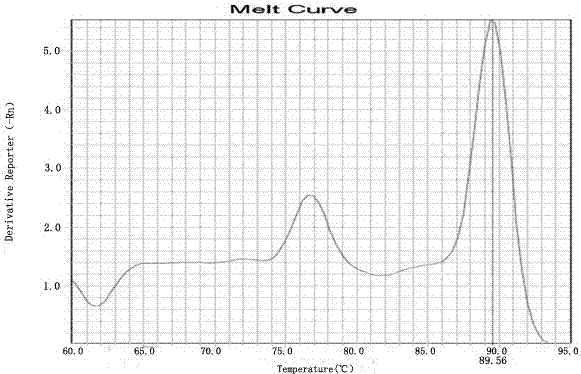
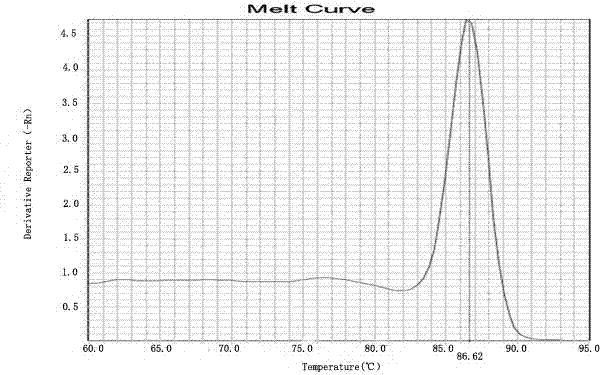
Kit for detecting HLA genotypes through fluorescent PCR melting curve assay
A melting curve and kit technology, which is applied in the field of kits for detection of HLA genotypes by fluorescent PCR melting curve method, can solve the problems that serology cannot be presented one by one, and there are many operation steps, so as to achieve the effect of quick result reference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. Preparation of reaction system
[0030] (1) Preparation of optical reaction plate
[0031] Take a 10mL volumetric flask, add 1500μL of 0.5OD / mL specific primer and 375μL of 0.5OD / mL internal quality control primer specified in the well according to the formula table of 96 wells, make up the volume to 10mL with sterile water, turn over to make it fully Mix well, transfer the liquid to a 15mL centrifuge tube, use the automatic pipetting workstation to dispense into the 96-well deep-well raw material plate, and then use the original plate-type dispensing workstation to dispense the primer mixture in the raw material plate into the optical reaction plate , after a brief centrifugation, dry in an environment with a temperature of 22±2°C and a humidity of 10±5%RH for 16-24 hours. After being sealed in a bag, the optical reaction plate can be used and stored at -20°C for later use.
[0032]
[0033]
[0034]
[0035] (2) Preparation of T3 reaction solution
[003...
Embodiment 2
[0054] 1. Reagent specificity verification
[0055] (1) Experimental samples
[0056] Six specific samples were taken to verify the specificity of the reagent, and the samples were purchased from the internationally recognized IHWG (http: / / www.ihwg.org / cellbank / ) standard.
[0057] (2) Experimental process
[0058] Use the HLA-ABDRDQ (fluorescence PCR melting curve method) nucleic acid detection kit to detect the above 6 specific samples respectively, analyze the detection results, and verify the specificity of the reagents.
[0059] (3) Experimental results
[0060] The test results of the 6 specific samples were all in line with expectations, indicating that the reagent has good specificity and no cross-reaction. The specific results are shown in the table below:
[0061] Specific test results
[0062]
[0063] 2. Reagent precision verification
[0064] (1) Experimental samples
[0065] One precision sample (concentration: 10ng / μL) was taken to verify the precision o...
Embodiment 3
[0085] Embodiment 3: Detect the result of 30 routine clinical samples
[0086] 1. According to the preparation method shown in Example 1, the relevant components of the kit were prepared and stored at -20°C for later use.
[0087] 2. Genomic DNA of 30 clinical samples was extracted using the nucleic acid extraction reagent (whole blood type) produced by Dobtech Biotechnology (Xiamen) Co., Ltd., and the purity of the DNA samples was tested with an ultraviolet spectrophotometer. The OD260 / OD280 of the 30 samples were all in the Between 1.6 and 2.0.
[0088] 3. According to the steps shown in Example 1, add DNA samples and perform detection on a fluorescent quantitative PCR instrument. The instrument used this time is ABI7500.
[0089] 4. According to the judgment standard shown in Example 1, the results are interpreted and counted, and the results are as follows:
[0090]
[0091]
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com