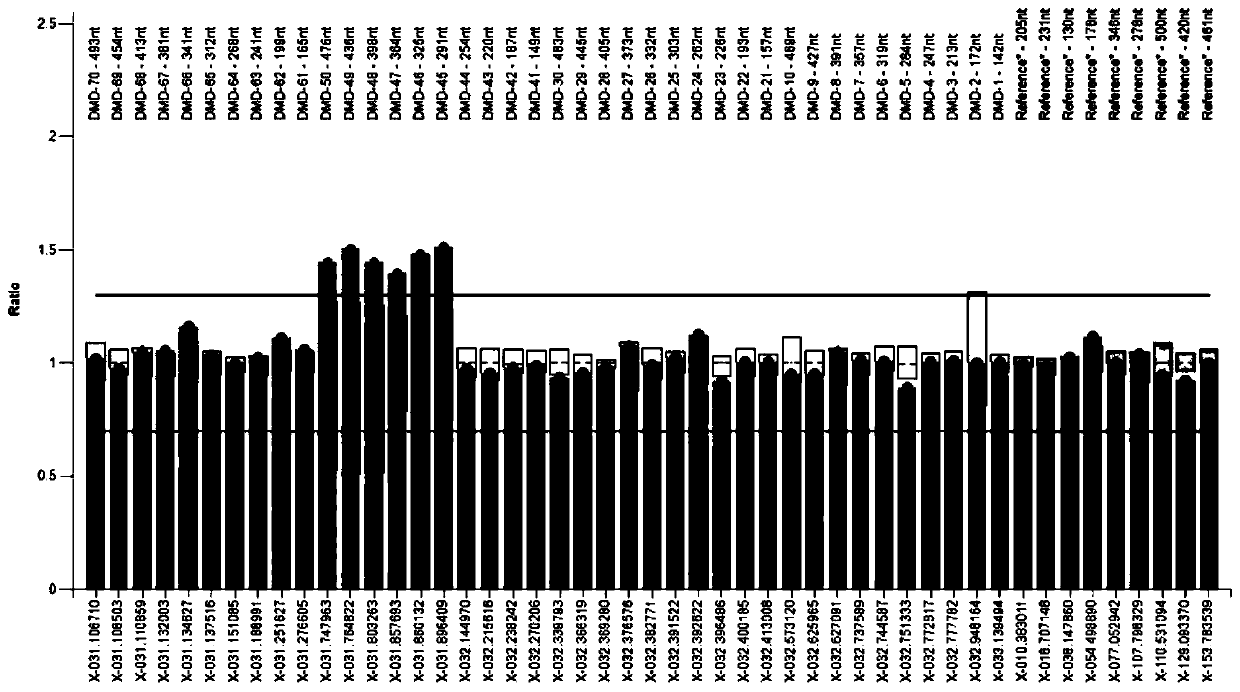
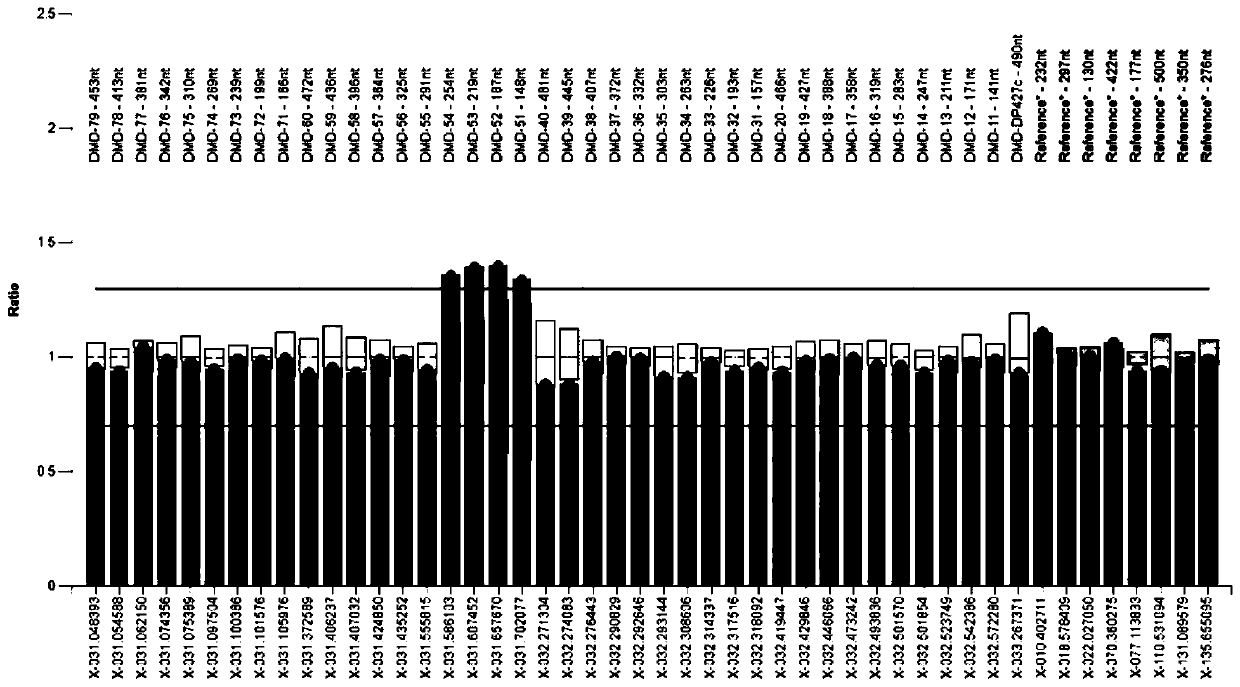
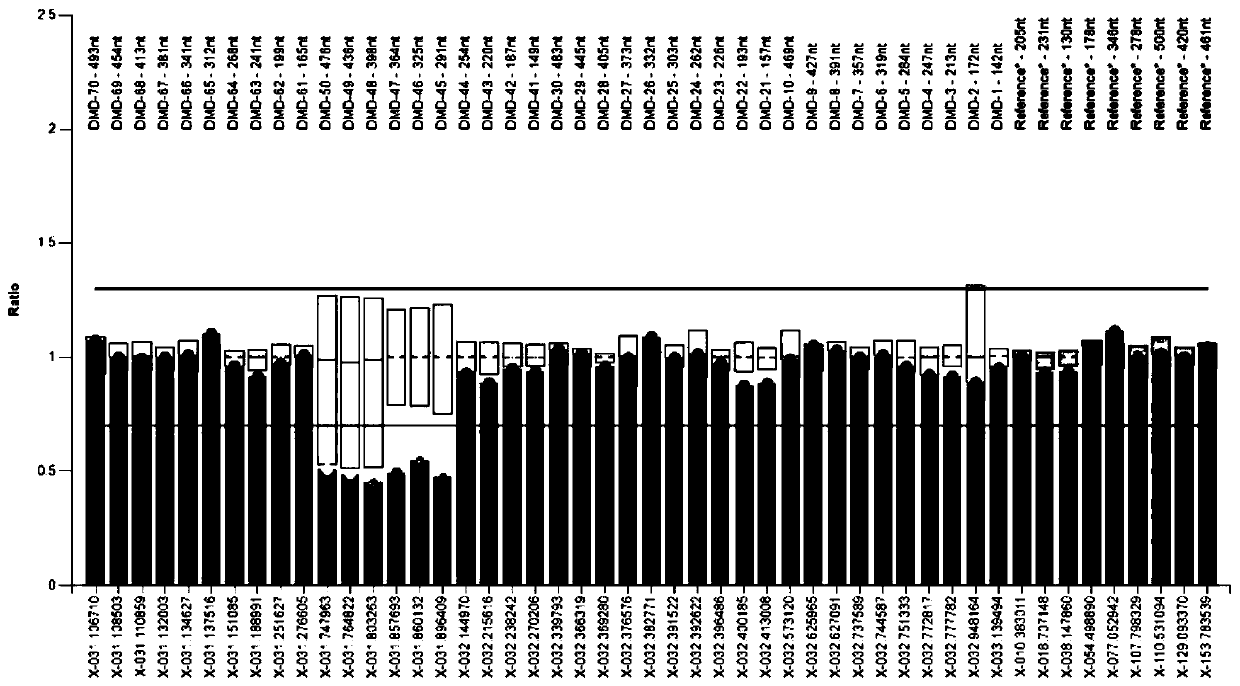
Amplifying system and reagent kit for detecting DMD (Duchenne muscular dystrophy) gene exon copy number variation
A technology of copy number variation and exon, which is applied in the field of amplification system and kit for detecting exon copy number variation of DMD gene, which can solve the problems of high price, high cost of reagents, fluctuations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] 1. Nucleic acid extraction:
[0092] 1 child with clinical diagnosis of DMD gave birth to 2 female carriers of children with clinical diagnosis of DMD, and also included whole blood samples from 3 normal females and 2 normal males. Use Tianlong Automatic Nucleic Acid Extractor (NP968-3S) and matching Tianlong Whole Blood Genomic DNA Extraction Kit to extract whole blood samples collected in EDTA anticoagulant tubes, and use a micro-ultraviolet spectrophotometer to determine the nucleic acid purity after extraction and concentration, its OD260 / OD280 is between 1.6-2.0; dilute the genomic DNA concentration with sterilized double distilled water to 20ng / μL for later use.
[0093] 2. Dilution of reference substance:
[0094] Take 10 μL of the first control and the second control respectively and add them to 2 eppendorffs, then add 10 μL of sterilized double distilled water, and mix well to obtain 20 μL each of the two 2-fold diluted control substances; 10 μL was taken out...
Embodiment 2
[0115] Reagent Specificity Validation: Cross-reactivity to Common Clinical Pathogens.
[0116] 1. Experimental sample:
[0117] Take 4 specific samples to verify the specificity of the reagent, hepatitis B virus (10 7 copies / ml), hepatitis C virus (10 5 copies / ml), human cytomegalovirus (10 4 copies / ml), Enterovirus 71 (10 4 copies / ml).
[0118] 2. Experimental process:
[0119] Use the first group of amplification composition reaction solutions to the tenth group of amplification composition reaction solutions to detect the above four specific samples respectively, analyze the detection results, and verify the specificity of the reagents.
[0120] 3. Experimental results:
[0121] The 4 specific samples tested by the ten kinds of reaction solutions were all Undetermined, indicating good specificity and no cross-reaction. The specific results are shown in Table 7 below.
[0122] Table 7: Results of cross-reaction between ten kinds of reaction solutions and common clinic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com