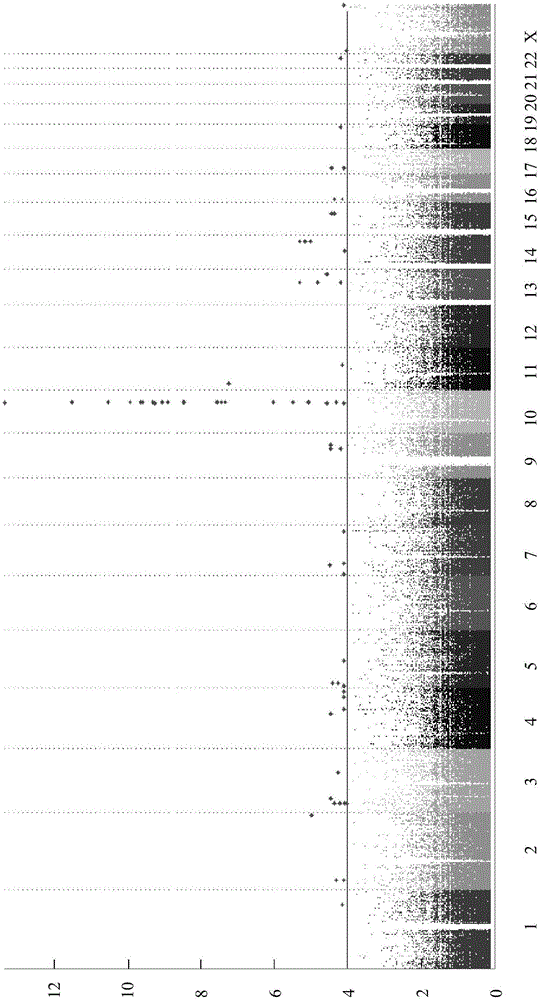
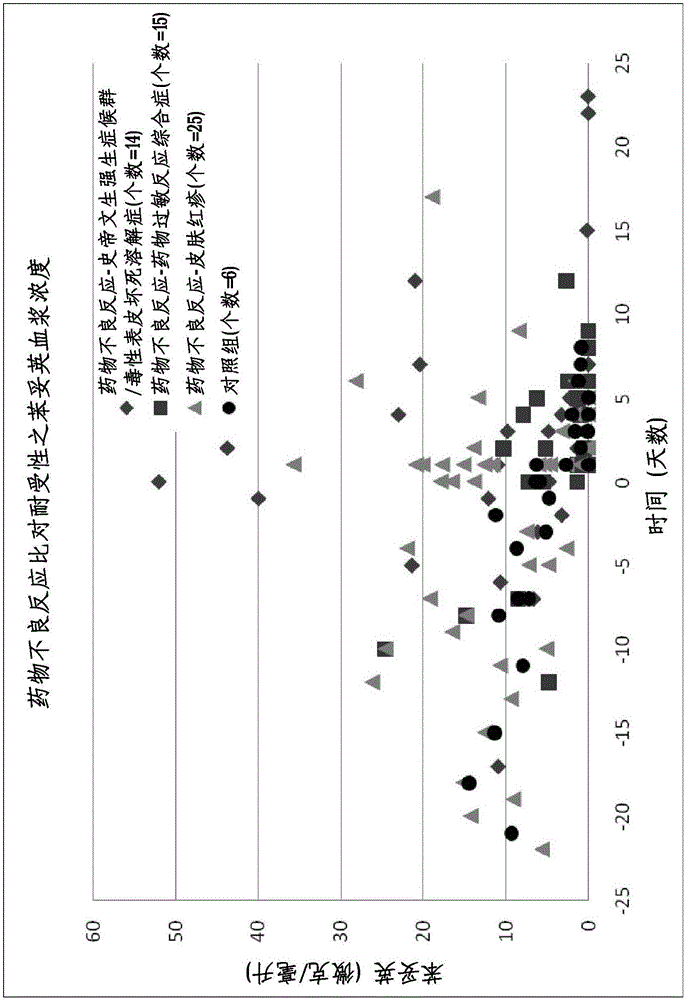
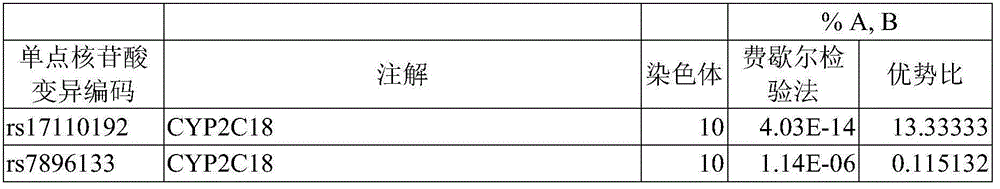
Method for evaluating drug anaphylactic reaction caused by antiepileptic drug phenytoin with HLA allele
A technology of alleles and HLA-B, applied in the direction of biochemical equipment and methods, measurement/testing of microorganisms, etc., can solve problems such as unclear relationships
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0011] The following detailed description is intended as a present exemplary description of aspects in accordance with the invention and is not intended to represent the only forms in which the invention may be made or utilized. It should be understood that the same or equivalent functions and elements that can be implemented in different embodiments also fall within the spirit and scope of the present invention.
[0012] Unless defined otherwise, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. Although any methods, devices and materials similar or equivalent to those described herein can be used in the practice or testing of the present invention, the exemplary methods, devices and materials are now described.
[0013] All publications mentioned are incorporated by reference for the purpose of describing and disclosing, for example, the designs and methodologies de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com