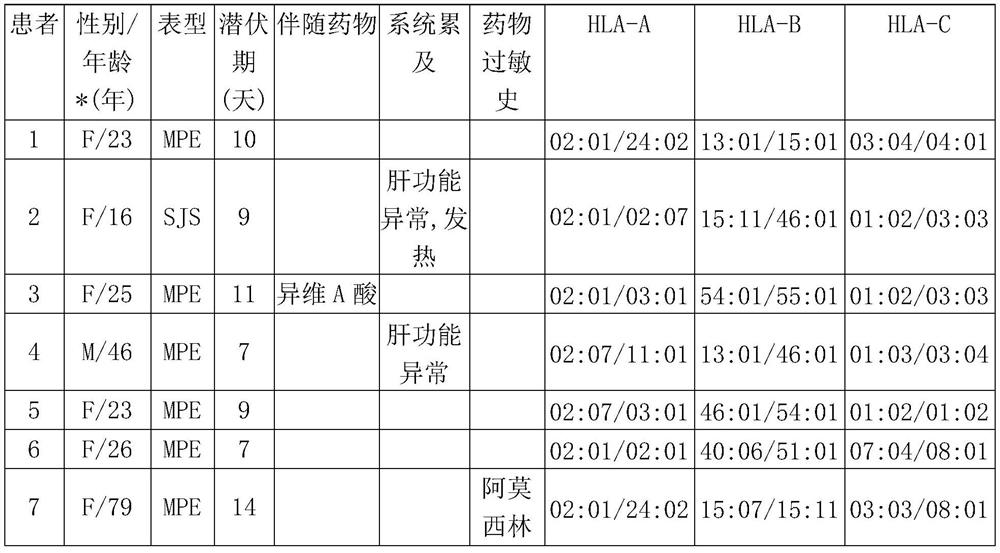
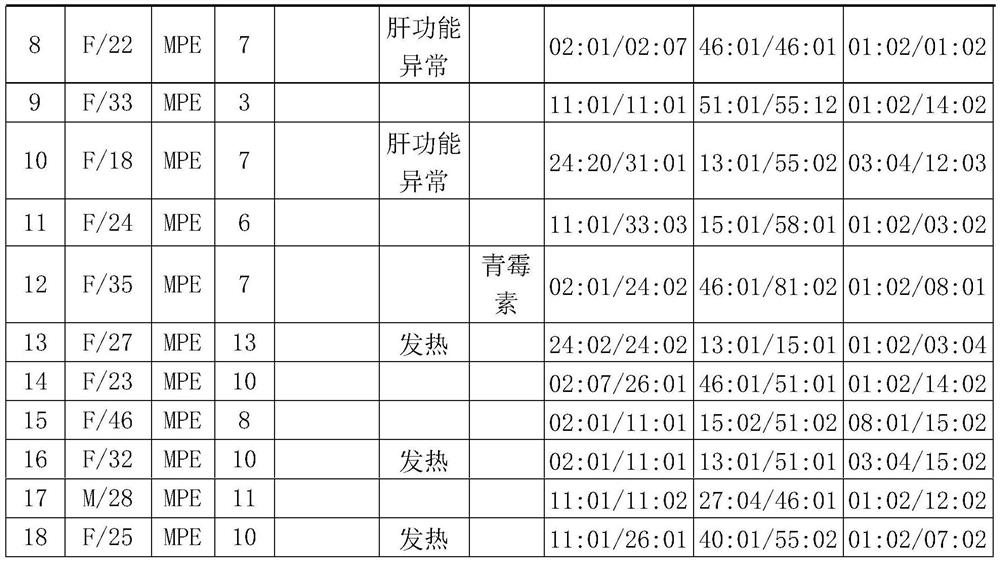
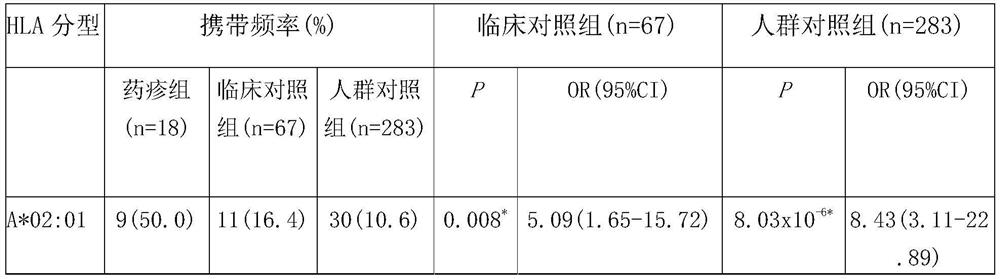
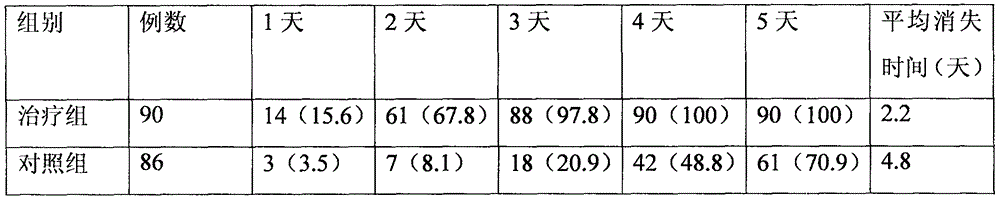
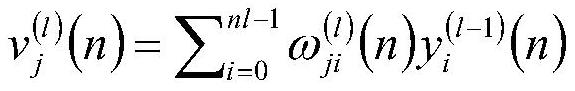Patents
Literature
33 results about "Drug rash" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
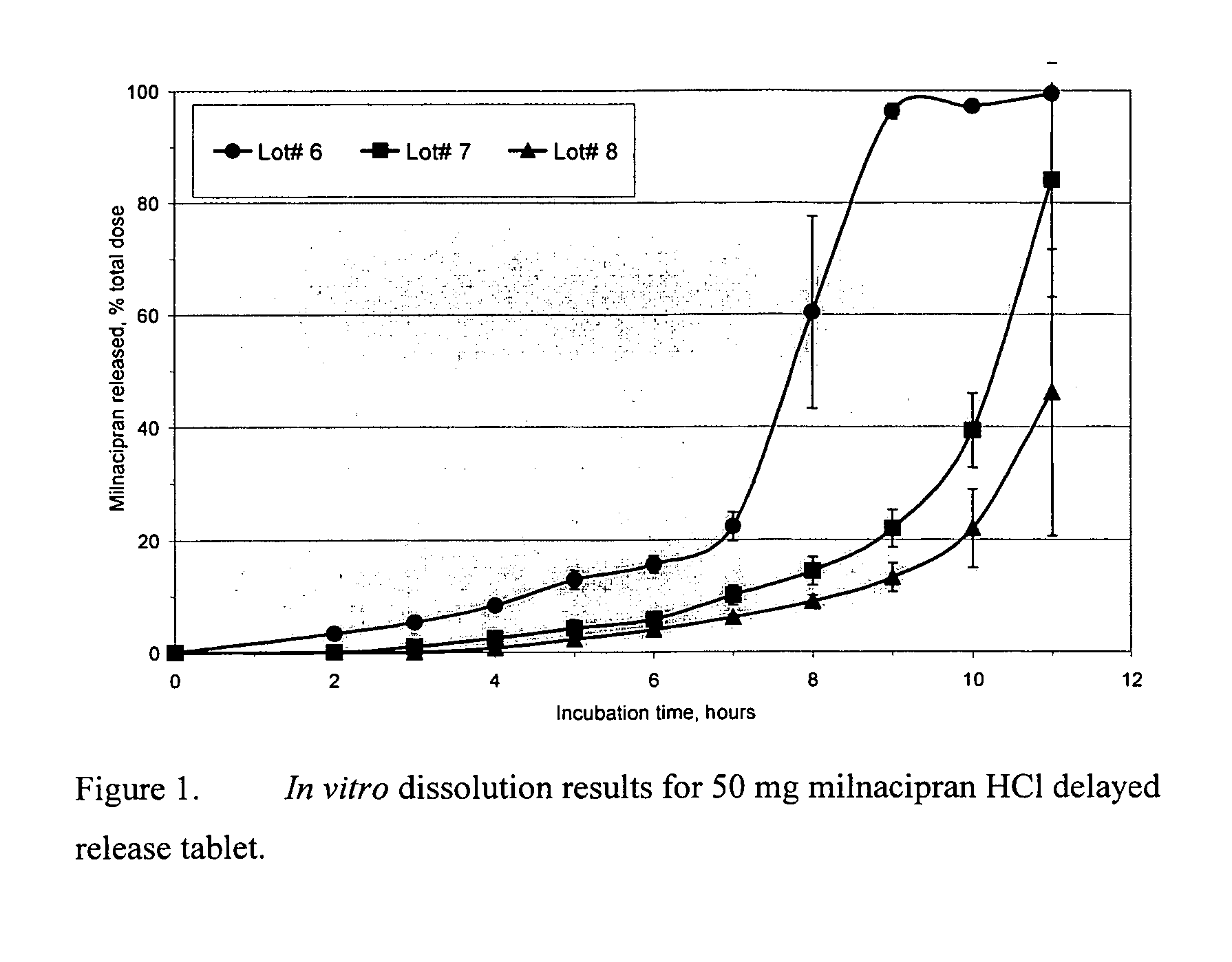
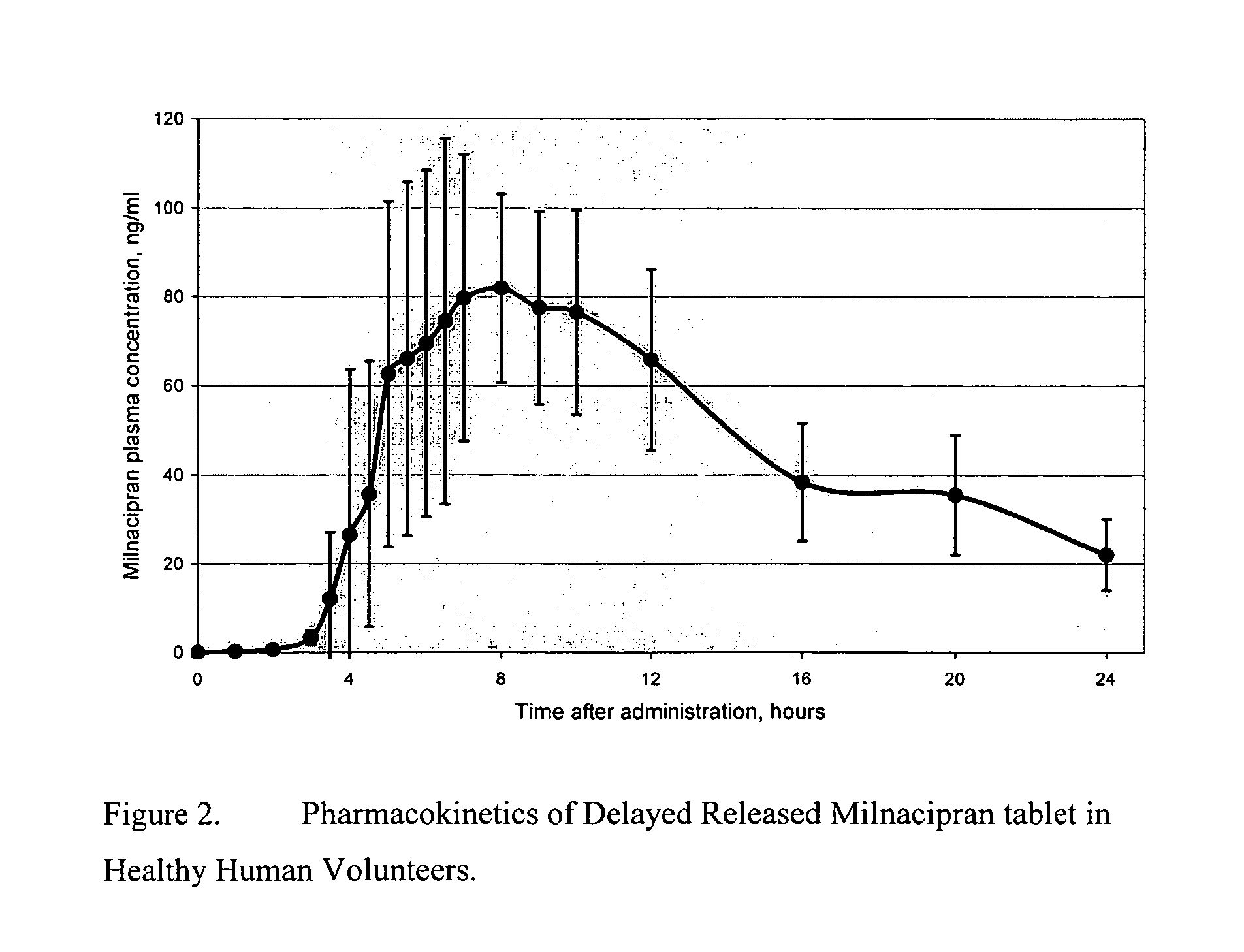
Pulsatile release compositions of milnacipran
InactiveUS20060003004A1Minimize exposureReduces milnacipran gastrointestinal side effectCapsule deliveryCoatingsPalpitationsPanic
A once-a-day oral milnacipran pulsatile release composition has been developed that releases the drug in spaced apart “pulses”. The dosage forms are comprised of first, second and optional third dosage units, with each dosage unit having a different drug release profile. This dosage form provides in vivo drug plasma levels characterized by Cmax below 3000 ng / ml, preferably below 2000 ng / ml, and most preferably below 1000 ng / ml. The composition provides pulsatile release of milnacipran to produce a therapeutic effect over approximately 24 hours, when administered to a patient in need, resulting in diminished incidence or decreased intensity of common milnacipran side effects such as sleep disturbance, nausea, vomiting, headache, tremulousness, anxiety, panic attacks, palpitations, urinary retention, orthostatic hypotension, diaphoresis, chest pain, rash, weight gain, back pain, constipation, vertigo, increased sweating, agitation, hot flushes, tremors, fatigue, somnolence, dyspepsia, dysoria, nervousness, dry mouth, abdominal pain, irritability, and insomnia.
Owner:COLLEGIUM PHARMA INC
Early stage wound healing using electromagnetic radiation
InactiveUS20030114884A1Speed upPresent methodElectrotherapyLight therapyPoison ivyElectromagnetic radiation
A device and method is disclosed for the treatment of early stage wounds, i.e. those wounds that have resulted in little or no breach of the skin tissue. The invention utilizes non-ablative laser or non-coherent electromagnetic radiation applied to a stage one or two wound to stimulate wound healing, destroy viral and bacterial bodies, and prevent the development of such wounds into higher stage wounds. An appropriate wavelength is chosen from the range of 193 nm to 10.6 microns, and is delivered at a power density of about at least 1 W / cm2 over a predetermined treatment duration typically in the range of 1 second to 3 minutes. To achieve the desired energy density, radiation is typically delivered at a power between 1 Watt and 15 Watts, with an average power of 5-10 Watts. Early stage wounds that can be addressed with this invention include but are not limited to spider or other insect bites, bee stings, rashes, eczema, psoriasis, and poison ivy. The present invention is especially useful for patients with a compromised ability to heal or stave off infection due to diabetes or other conditions.
Owner:BIOLITEC PHARMA MARKETING
Drugs for desensitizing rashes and sores
InactiveCN102266486AReduce permeabilityStabilize cell membraneImmunological disordersDermatological disorderAllergic dermatitisDisease
The invention relates to a medicine for desensitizing, eliminating rashes and sores. The medicine for desensitizing, eliminating rashes and sores of the present invention is to take 20-40g of Radix Astragali, 20-40g of Radix Glycyrrhizae, 15-35g of Polygonum Polygoni Multiflori, 15-35g of Polygonatum Polygonatum, 10-20g of Morinda officinalis, and dry the traditional Chinese medicines of the above-mentioned parts by weight, Then grind it into a fine powder, add 100~200g of Vaseline, mix well, and prepare it. The medicine for desensitizing rashes and sores of the present invention is obtained through many years of clinical experience. It has a unique curative effect on chronic diseases, and the effect is better than western medicine, and it has been tried and tested.
Owner:陈忠和
Medicament for treating rash
InactiveCN101966329AColor back to normalLittle side effectsAnthropod material medical ingredientsDermatological disorderDiseaseSide effect
The invention relates to a medicament, in particular to a medicament for treating rash, which is prepared from various traditional Chinese medicines serving as raw materials, and mainly solves the problem that the conventional medicament for treating rash has effect of curing symptoms but not root causes, causes recrudescence, has large side effects, is easy to generate medicament resistance, has poor curative effect and the like. The medicament consists of pseudoginseng root, figwort root, great burdock achene, dendrobium, coastal glehnia root, Chinese dwarf cherry seed, dwarf lilyturf tuber, fragrant solomonseal rhizome, bupleurum, baical skullcap root, Chinese gentian, natural indigo, talcpowder, asiatic moonseed rhizome, blackberrykiky rhizome, rehmannia, cortex moutan, green tangerine peel, cicada shell, hawthorn, stiff silkworm, Mongolian snakegourd root, indigowoad leaf, liquorice, Chinese date and ginger according to a certain ratio. The medicament is prepared from the traditional Chinese medicines to treat and condition the disease, treats both the symptoms and the root causes, and has the advantages of small side effect, no medicament resistance after long-term administration, no recrudescence, obvious curative effect and high cure rate.
Owner:安政权
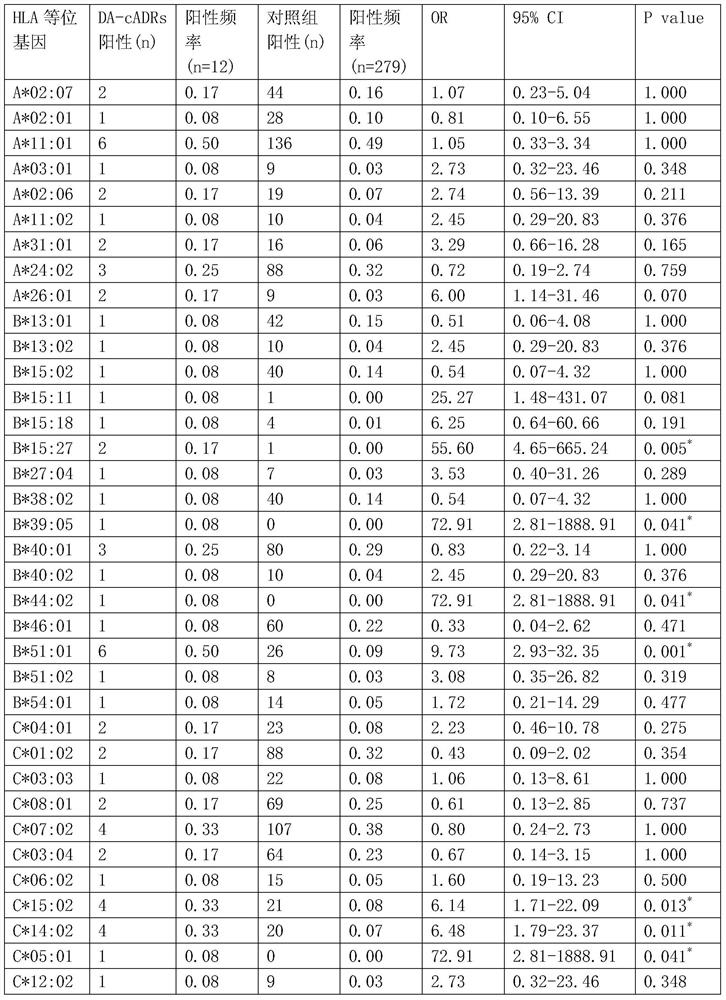
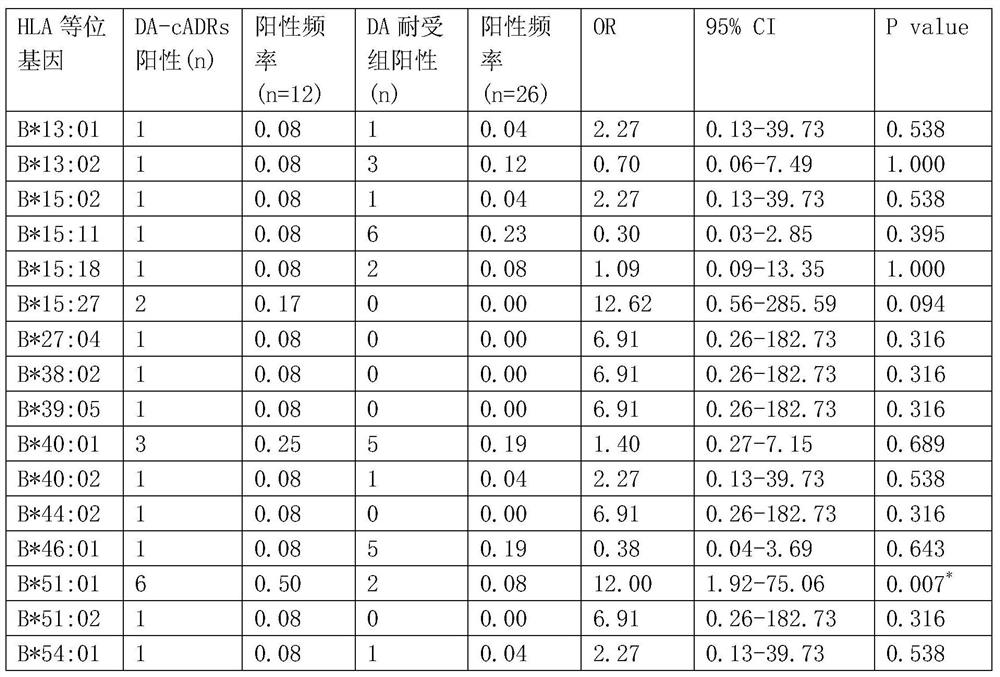
Human leukocyte antigen gene detection kit for screening clindamycin-induced adverse drug reactions on skin
ActiveCN106755291ABlocking interactionMicrobiological testing/measurementBiological material analysisAntigenMetabolite
The invention belongs to the technical field of biological medicine, and relates to a human leukocyte antigen HLA-B*51: 01 gene detection kit. An HLA-B*51: 01 gene can be used as a marker gene for predicting clindamycin-induced drug rash. The invention provides the application of the detection kit to typing of the HLA-B*51: 01 gene in a sample. The application comprises the following steps: extracting DNA from obtained peripheral blood of a patient, and detecting the HLA-B*51: 01 gene by a current method, such as a sequence-specific oligonucleotide probe (PCR-SSO) method. The HLA-B*51: 01 gene detection kit provided by the invention can be applied to screening and sifting of targeted drugs for treating the clindamycin-induced drug rash before the use of clindamycin; screening application can guide clinical drug use so as to reduce incidence of the clindamycin-induced drug rash; selecting application mainly acts on an HLA-B*51: 01 molecule in a targeted manner so as to block interaction between the HLA-B*51: 01 molecule and clindamycin or other metabolites during the drug rash.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Traditional Chinese drug antipyretic pills
InactiveCN102233121AImprove drug toleranceEasy access to medicinal ingredientsAntipyreticAnalgesicsPinus koraiensisHypersensitive response
The invention discloses traditional Chinese drug antipyretic pills, and relates to the technical field of traditional Chinese medicine. The traditional Chinese drug antipyretic pills provided by the present invention comprise the following raw materials, by weight, 1-50 parts of an extract of a component A, 1-50 parts of curcuma powder, 50-200 parts of cape jasmine powder and 50-200 parts of coptis chinensis powder, wherein the component A comprises the raw materials of pinus koraiensis branches, birch branches and poplar branches, wherein a weight ratio of the pinus koraiensis branches to the birch branches to the poplar branches is 1:1-4:1-4. The traditional Chinese drug antipyretic pills have effects of convulsion relieving and resuscitation, fever removing and heat clearing. In addition, after taking the traditional Chinese drug antipyretic pills, patients have good drug tolerance; gastrointestinal reactions are not generated; liver function indexes and kidney function indexes show no abnormal before and after taking the traditional Chinese drug antipyretic pills; anaphylactic responses such as erythra and the like are not generated; the traditional Chinese drug antipyretic pills have rapid onset and a short course of treatment.
Owner:DALIAN SHENZHI KANGYUAN TECH
Biomarkers for serious skin rash
The present invention provides a method for predicting the risk of a patient for developing adverse drug reactions, particularly Serious Skin Rash (SSR), including such severe adverse reactions such as Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN). The invention also provides a method of identifying a subject afflicted with or at risk of developing SSR. In some aspects, the methods comprise analyzing at least one genetic marker, wherein the presence of the at least one genetic marker indicates that the subject is afflicted with or at risk of developing SSR. Genetic markers useful in accordance with the methods of the invention are disclosed.
Owner:FLORATOS ARIS +2
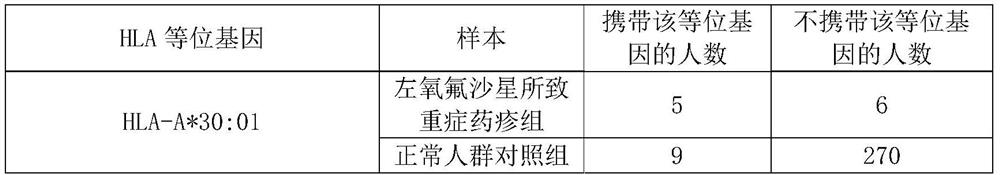
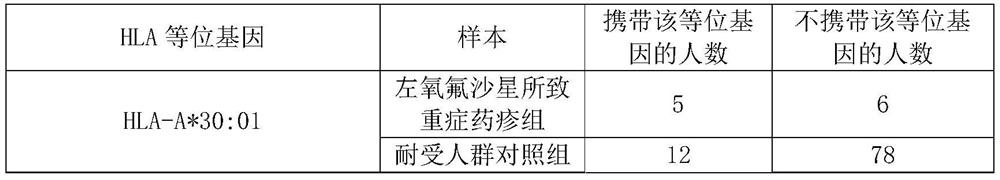
Application of substance for detecting HLA-A*30:01 allele in evaluating risk of severe drug rash caused by levofloxacin
ActiveCN112029847AReduce generationMicrobiological testing/measurementAgainst vector-borne diseasesWhite blood cellBiology
The invention discloses an application of a substance for detecting an HLA-A*30:01 allele in evaluating the risk of human severe drug rash caused by levofloxacin. The invention also discloses an application of the substance for detecting the HLA-A*30:01 allele in preparation of a product for detecting or evaluating the risk of human adverse drug reaction responding to the levofloxacin. Experimentsprove that the human leukocyte antigen gene-HLA-A*30:01 allele is related to the risk of the severe drug rash caused by the levofloxacin. The HLA-A*30:01 allele can be used as a marker gene for predicting the occurrence risk of the severe drug rash caused by the levofloxacin.
Owner:FUDAN UNIV +1
Medicated diaper
An anti-rash diaper for preventing and treating diaper rash and skin irritations comprising: an absorbent layer, where the absorbent layer draws moisture; medicated ointment, where the ointment is applied over the absorbent layer; and a protective sheet, where the protective sheet is releasably attached over the ointment. The anti-rash diaper according to the present invention may further include elastic waistbands. A hook and loop fastener may be used to attach the protective sheet to the diaper. The protective sheet effectively secures the ointment over the absorbent layer.
Owner:AKINSANYA ADERONKE
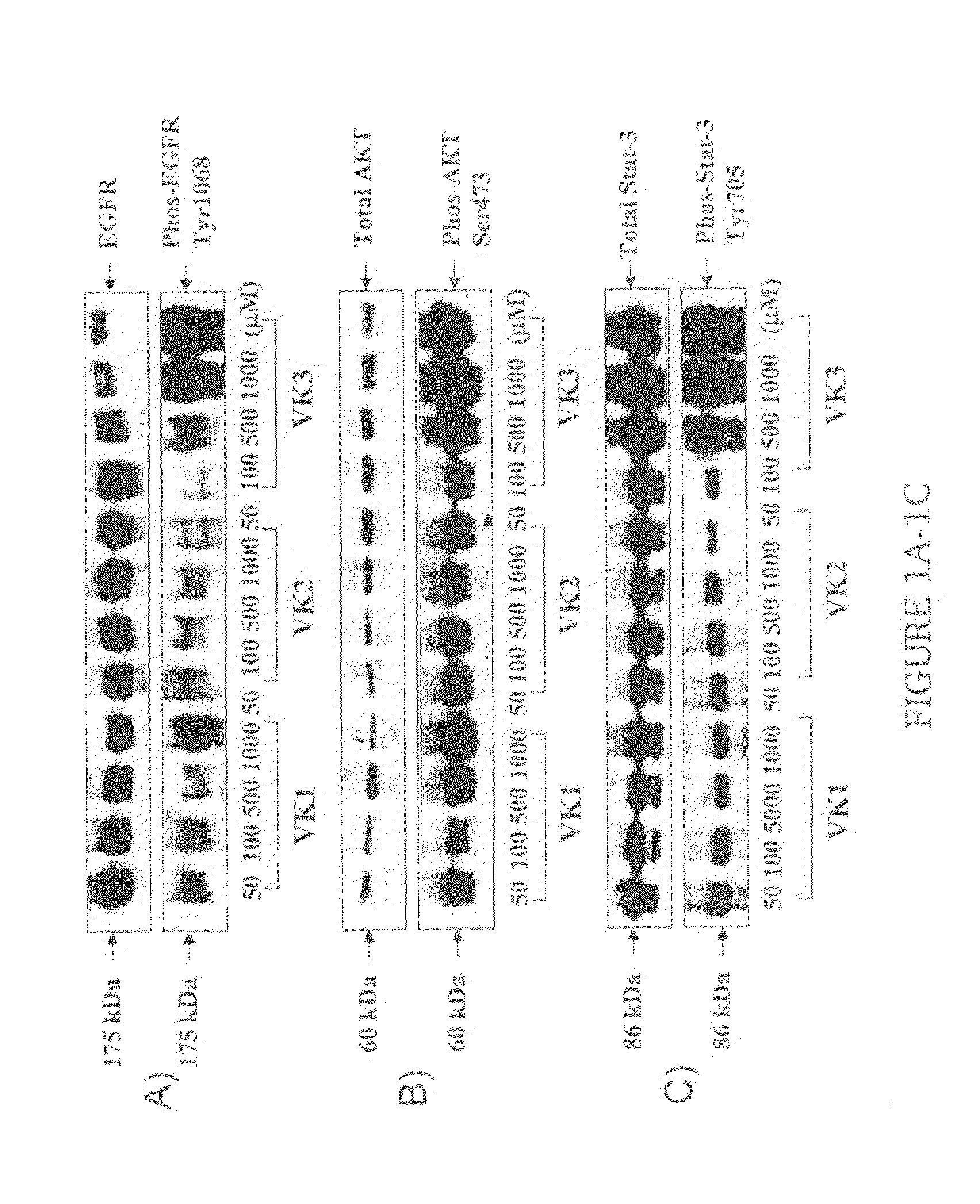
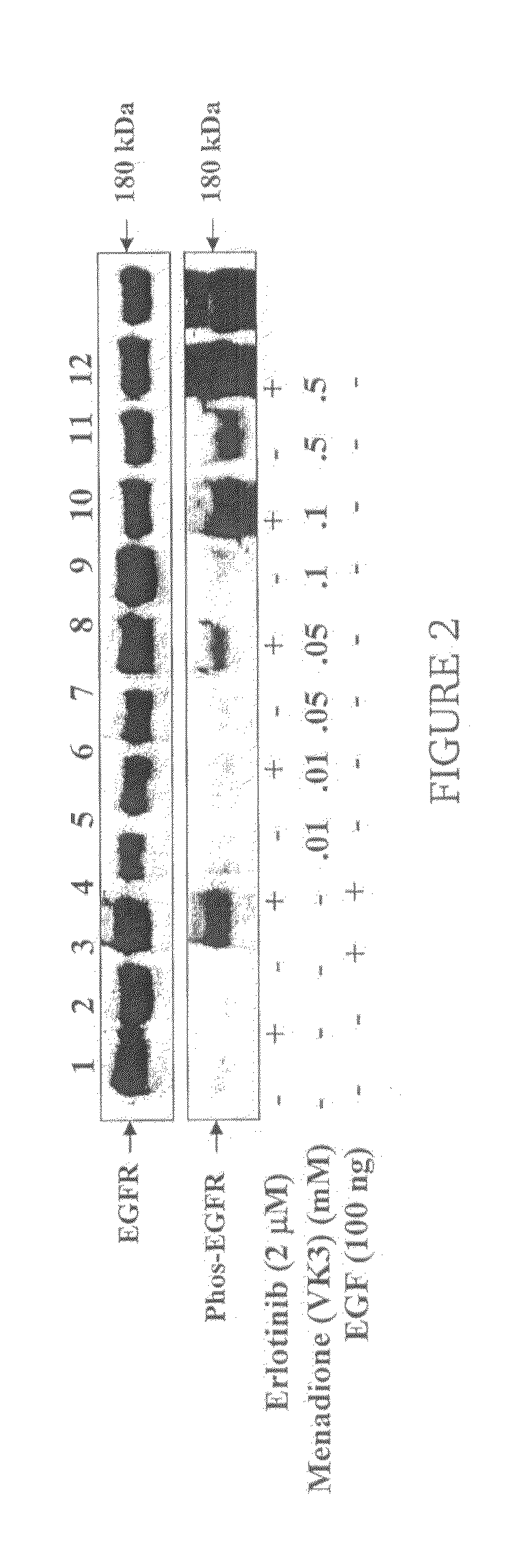
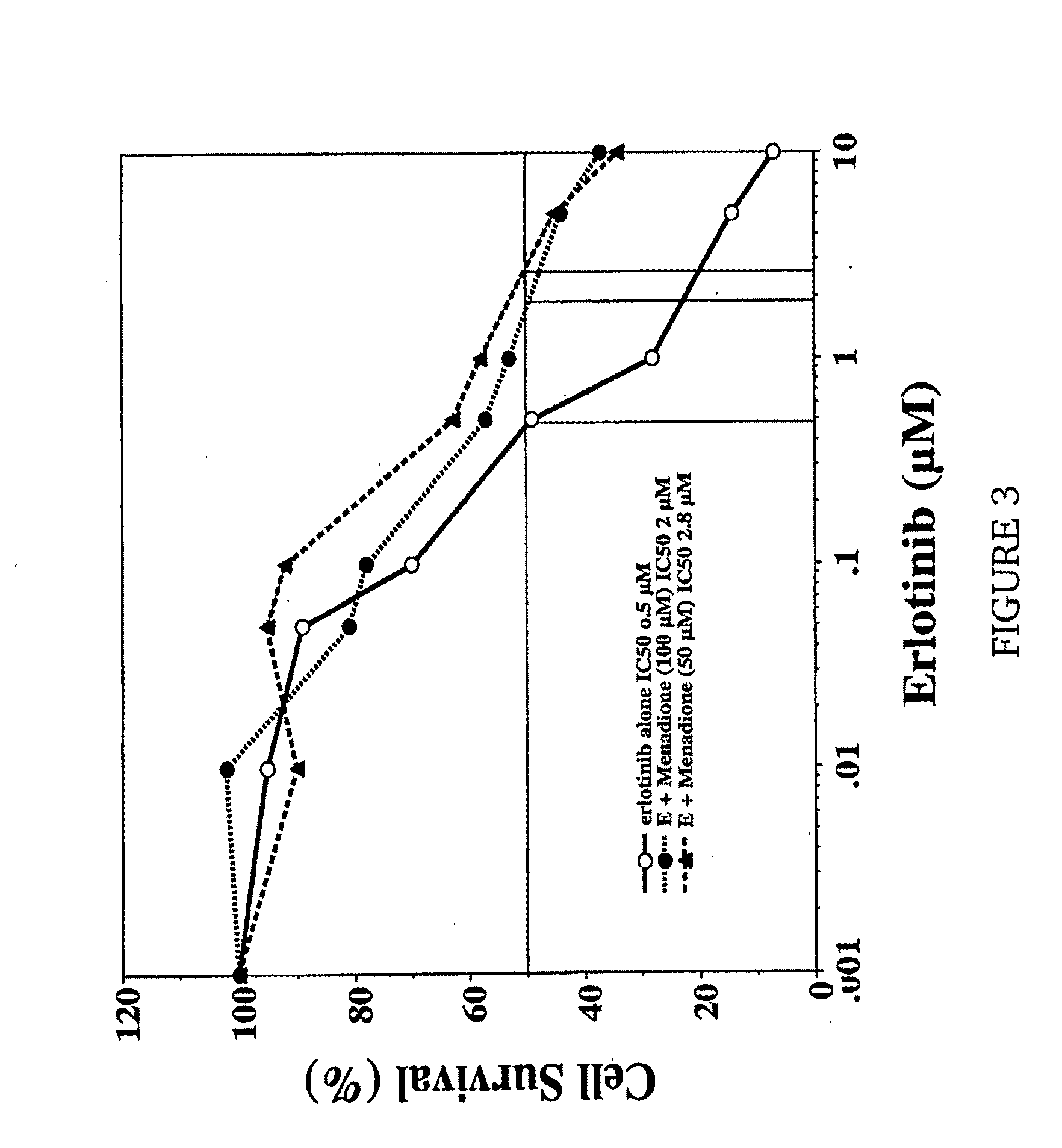
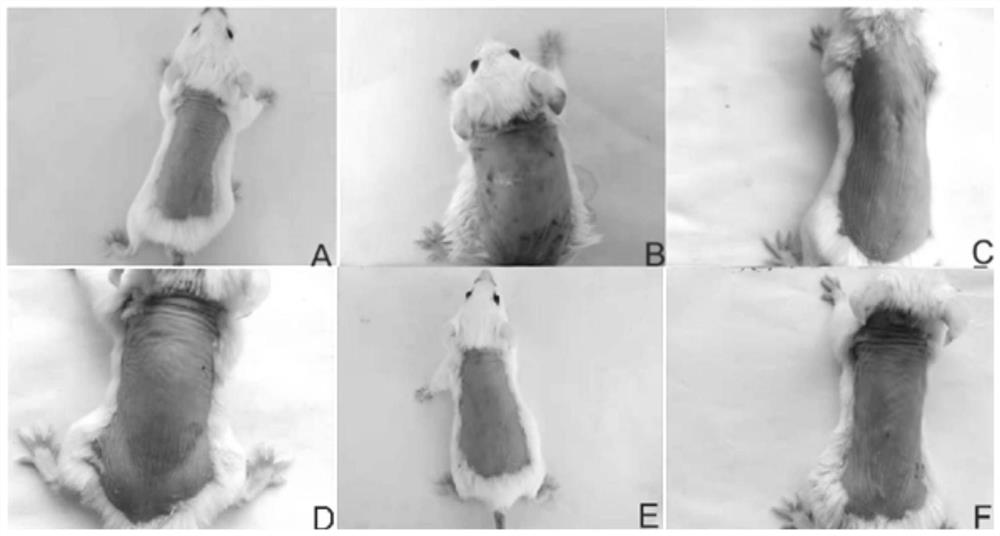
Vitamin K for Prevention and Treatment of Skin Rash Secondary to Anti-EGFR Therapy
The invention provides methods and compositions for treating and preventing a skin rash secondary to anti-epidermal growth factor receptor (EGFR) therapy, where the method comprises applying a vitamin K analog or a phosphatase inhibitor to the skin.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE INC
Application of fraxinellone in preparation of drugs for treating allergic skin diseases
InactiveCN113456628APromote productionOrganic active ingredientsImmunological disordersDiseaseContact dermatitis
The invention discloses an application of fraxinellone in preparation of drugs for treating allergic skin diseases. The allergic skin diseases comprise urticaria, eczema, contact dermatitis, atopic dermatitis, anaphylactoid purpura and drug rash. According to the invention, fraxinellone is used for treating immune globulin E (IgE)-mediated skin diseases for the first time, and fraxinellone can promote Th1 type cells to generate IFN-gamma. 25 mg / kg of fraxinellone is given to mice with passive skin allergy, degranulation of skin tissue mast cells is inhibited, and the inhibition rate reaches 34.01%. 50 mg / kg of fraxinellone is given to mice with passive skin allergy, and the degranulation inhibition rate of skin mast cells reaches 54.34%. 100 mg / kg of fraxinellone is given to mice with passive skin allergy, and the degranulation inhibition rate of skin mast cells reaches 62.5%.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Traditional Chinese medicine composition for externally applying to treat benign prostatic hyperplasia
ActiveCN104474457AReduce adverse reactionsPharmaceutical delivery mechanismMedical devicesDiseaseSalvia miltiorrhiza
The invention relates to a traditional Chinese medicine composition for externally applying to treat benign prostatic hyperplasia. The traditional Chinese medicine composition is prepared from the following medicinal raw materials by weight: 450-550g of angelica sinensis, 450-550g of sichuan lovage rhizome, 450-550g of flos carthami, 450-550g of peach kernels, 450-550g of astragalus membranaceus, 450-550g of salviae miltiorrhizae, 450-550g of curcuma aromatica, 450-550g of cortex moutan and 450-550g of honeysuckle. The traditional Chinese medicine composition provided by the invention is suitable for crowds of all age groups and free of any influence on crowds with background diseases. Although the traditional Chinese medicine composition is prepared from common traditional Chinese medicines, the effect is still remarkable if the traditional Chinese medicine composition is used in an insisted manner. Under the joint effect with infrared rays, the efficacy is absorbed by virtue of skin vessels and the composition can be directly acted on the prostate. The most major advantage of the traditional Chinese medicine composition is to reduce adverse reactions caused by long-term administration of the patient. Except for a small part of patients with rash itch, no other obvious side effects are available, and the clinical follow-up survey effective rate is over 98%.
Owner:界首骨科医院
Traditional Chinese herbal medicine qi-tonifying and blood-nourishing food additive and preparation method thereof
The invention relates to a traditional Chinese herbal medicine qi-tonifying and blood-nourishing food additive and a preparation method thereof, and relates to a medicine preparation comprising traditional Chinese medicines. The invention aims to provide the traditional Chinese herbal medicine qi-tonifying and blood-nourishing food additive with quick response, high effective rate and no side effect during long-time use, and the preparation method thereof. The traditional Chinese herbal medicine qi-tonifying and blood-nourishing food additive comprises the following components in parts by mass: 3 to 7 parts of fructus schizandrae, 3 to 7 parts of radix polygoni multiflori, 2 to 6 parts of fructus ligustri lucidi, 4 to 8 parts of folium nelumbinis, 5 to 9 parts of walnuts, 3 to 7 parts of radix ginseng, 4 to 8 parts of rhizoma atractylodis macrocephaiae, 8 to 12 parts of radix astragali and 8 to 12 parts of donkey-hide gelatin. The traditional Chinese herbal medicine qi-tonifying and blood-nourishing food additive can be added into beverage, food, supplements or breeding feed for long-time use, and avoids side effects of fever, drug rash, nausea, diarrhea and the like.
Owner:陈庄星
Drug rash plasma exosome circRNA marker and detection primer kit thereof
PendingCN112921077AAccurate judgmentImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationDiseaseDrug rash
The invention discloses a drug rash plasma exosome circRNA marker. The drug rash plasma exosome circRNA marker comprises a primer and a probe of a combination of a drug rash plasma exosome long RNA marker and a miRNA marker; the long RNA marker comprises one or more of circ_0047921, hsa_circ_0006773 or circ_0056285; and the miRNA marker comprises one or more of miR-375-3p, miR-200a-3p, miR-122-5p and miR-27a-3p. The invention provides the drug rash plasma exosome circRNA marker; after clinical verification, the drug rash plasma exosome circRNA marker has relatively high specificity and sensitive value, and has relatively high diagnostic reference value; by analyzing the expression condition of the circRNA in the plasma exosome, whether a subject suffers from drug rash or not can be accurately judged with a large probability; a basis is provided for early discovery of diseases; opportunities are provided for early treatment of patients; the diagnosis accuracy is improved; the diagnosis process is simplified, and the invention has a large application prospect.
Owner:AIR FORCE MEDICAL UNIV
Biological agent formula for facilitating skin wound healing and preparation method thereof
InactiveCN105497287APromote growthPromote regenerationUnknown materialsPharmaceutical non-active ingredientsBiocompatibility TestingToxic material
The invention provides a biological agent formula for facilitating skin wound healing and a preparation method thereof. The biological agent is prepared from lithospermum, radix angelicae, sanguisorba officinalis, Chinese angelica, Astragalus membranaceus, Kochia scoparia, honeysuckle, Scutellaria baicalensis, tea oil and beeswax. The method comprises the following steps of A powdering and grinding, B, herbal medicine preparation and C storing. Compared with the prior art, the biological agent has the advantages of relieving heat and removing toxic materials, removing the necrotic tissue and promoting granulation, clearing away heat and toxic materials, cooling blood to relieve pain, and being used for eczema, dermatitis, drug rash, scalding, anabrosis and the like, has the flexible performance similar to that of the human skin, can keep the certain form and strength when wetted, promotes granulation growth and skin regeneration, accelerates healing, reduces scars, and is good in biocompatibility and easy to degrade.
Owner:马玉玲
Human leukocyte antigen gene detection kit for screening skin adverse reactions caused by clindamycin
ActiveCN106755291BBlocking interactionMicrobiological testing/measurementBiological material analysisAntigenMetabolite
The invention belongs to the technical field of biological medicine, and relates to a human leukocyte antigen HLA-B*51: 01 gene detection kit. An HLA-B*51: 01 gene can be used as a marker gene for predicting clindamycin-induced drug rash. The invention provides the application of the detection kit to typing of the HLA-B*51: 01 gene in a sample. The application comprises the following steps: extracting DNA from obtained peripheral blood of a patient, and detecting the HLA-B*51: 01 gene by a current method, such as a sequence-specific oligonucleotide probe (PCR-SSO) method. The HLA-B*51: 01 gene detection kit provided by the invention can be applied to screening and sifting of targeted drugs for treating the clindamycin-induced drug rash before the use of clindamycin; screening application can guide clinical drug use so as to reduce incidence of the clindamycin-induced drug rash; selecting application mainly acts on an HLA-B*51: 01 molecule in a targeted manner so as to block interaction between the HLA-B*51: 01 molecule and clindamycin or other metabolites during the drug rash.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Medicine for treating acute urticaria
InactiveCN1615951ARash subsidesLow cost of treatmentAnthropod material medical ingredientsImmunological disordersDiseaseSide effect
The medicine for treating acute urticaria is oral liquid preparation prepared with eight kinds of Chinese medicinal materials, including long-noded pit viper, chrysanthemum, cicada slough, isatis root, ledebouriella root, etc. in certain proportion. The medicine of the present invention can cure urticaria radically, and has high curative effect, less toxic side effect, and low cost.
Owner:尹宇清
Traditional Chinese medicine injection for treating malaria
InactiveCN106389500AQuick resultsImprove drug tolerancePharmaceutical delivery mechanismAntiparasitic agentsDiseaseTolerability
The invention discloses a traditional Chinese medicine injection for treating malaria, and relates to the technical field of traditional Chinese medicine. The traditional Chinese medicine injection for treating malaria comprises the following raw materials in parts by weight: 100 to 1000 parts of southernwood, 100 to 1000 parts of inular flowers and 1000 to 2000 parts of water. The prepared medicine has the advantages that the effects of clearing away heat and toxic materials, strengthening body resistance and eliminating evil are achieved; the malaria diseases can be treated; in addition, after the use of the medicine, the patient has good medicine tolerance and does not have allergic reaction such as eruption; symptoms of gastro-intestinal comfortlessness, diarrhea and the like cannot occur in the medicine use period; the effect taking speed is high; the treatment coarse is short.
Owner:威海恒基伟业信息科技发展有限公司
Pharmaceutical composition with pure plant origin and application thereof
InactiveCN101584759BReduce dosageReduce recurrenceDermatological disorderPlant ingredientsBacillus acnesPlant Sources
The invention provides a pharmaceutical composition with a pure plant origin and application thereof. Pharmacological studies show that the medicament can treat acne from multiple aspects and ensure that propionibacterium acnes do not generate drug resistance. Clinical experiment shows that the medicament has quick response, can cure the acne in a short time, obviously reduces the recurrence, hasgood safety and does not have obvious adverse reaction. Compared with single use of a tanshinone extract (such as a tanshinone capsule), the medicament reduces the dosage, improves the treatment effect and avoids the allergic reaction, such as rash, lip rash and the like. Simultaneously the quality of the medicament and the stability of the healing efficacy are ensured by effectively controlling active ingredients.
Owner:中北科技集团股份有限公司
Application of dendrophenol and composition thereof in preparation of medicine for treating allergic dermatosis
ActiveCN114652706ASignificant progressObvious advantagesEther/acetal active ingredientsImmunological disordersDiseaseChlorobenzene
The invention discloses application of dendrophenol and a composition thereof in preparation of drugs for treating allergic dermatosis, and belongs to the technical field of new medical application of active component dendrophenol of traditional Chinese medicinal materials. In particular to application of dendrophenol in preparation of drugs for treating allergic skin diseases, and the allergic skin diseases comprise urticaria, eczema, contact dermatitis, atopic dermatitis, anaphylactoid purpura and drug rash. Animal test results show that the dendrophenol can inhibit inflammatory response, ear swelling and scratching times of a guinea pig allergic skin disease model induced by 2, 4-dinitrochlorobenzene, and reduce infiltration of eosinophilic granulocytes and mast cells at an affected part, so that the dendrophenol has the effects of resisting allergic reaction, diminishing swelling and relieving itching, and has a very good treatment effect on allergic skin diseases.
Owner:HEFEI NORMAL UNIV
Medicine for treating acute urticaria
InactiveCN1284554CRash subsidesLow cost of treatmentAnthropod material medical ingredientsReptile material medical ingredientsDiseaseSide effect
The medicine for treating acute urticaria is oral liquid preparation prepared with eight kinds of Chinese medicinal materials, including long-noded pit viper, chrysanthemum, cicada slough, isatis root, ledebouriella root, etc. in certain proportion. The medicine of the present invention can cure urticaria radically, and has high curative effect, less toxic side effect, and low cost.
Owner:尹宇清
Application of HLA-C*02:01:01 alleles in detection or evaluation of adverse drug reactions of efavirenz
PendingCN113881762AHigh hazardMicrobiological testing/measurementDNA/RNA fragmentationDrug adverse reactionsDrug utilisation
The invention discloses application of HLA-C*02:01:01 alleles in detection or evaluation of adverse drug reactions of efavirenz, and relates to the technical field of biological medicines. Specifically, the invention discloses application of a substance for detecting whether a person has HLA-C*02:01:01 alleles or not in preparation of a product for detecting or evaluating the risk that the person has adverse drug reactions responding to efavirenz. The invention provides marker genes, namely the HLA-C*02:01:01 alleles, which can be used for detecting or evaluating the occurrence risk of adverse drug reactions responding to efavirenz. By detecting whether the to-be-detected person carries the HLA-C*02:01:01 alleles or not, the high-risk person with efavirenz-induced drug rash can be screened; and the application is used for guiding clinical medication, so that occurrence of efavirenz-induced drug rash is reduced.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Application and detection reagent for screening allopurinol tolerant gene methylation marker
PendingCN114457149AIncreased sensitivityImprove featuresMicrobiological testing/measurementITGB2 GeneMedicine
The invention relates to application and a detection reagent for screening allopurinol tolerant gene methylation markers. The marker comprises an HLA-B * 58: 01 allele, and methylation level detection of HLA-B gene, TRAF1 gene, ITGB2 gene, BCL2 gene, ITGB2-AS1 gene, REPIN1 gene, JPH3 gene, KCNJ1 gene, METTL11B gene and ACSL-1 gene. The method is mainly used for evaluating the risk that a subject suffers from severe drug rash caused by allopurinol, and the sensitivity and specificity of detecting and screening the severe drug rash caused by allopurinol through pure HLA-B * 58: 01 allele are remarkably improved; particularly, the method has important clinical significance on identification of purinol tolerant population with positive HLA-B * 58: 01 allelic genes and intolerant population with negative HLA-B * 58: 01 allelic genes. The method is simple and convenient to operate, high in detection specificity and sensitivity, short in time consumption, small in required sample amount, easy to clinically popularize and wide in application prospect.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
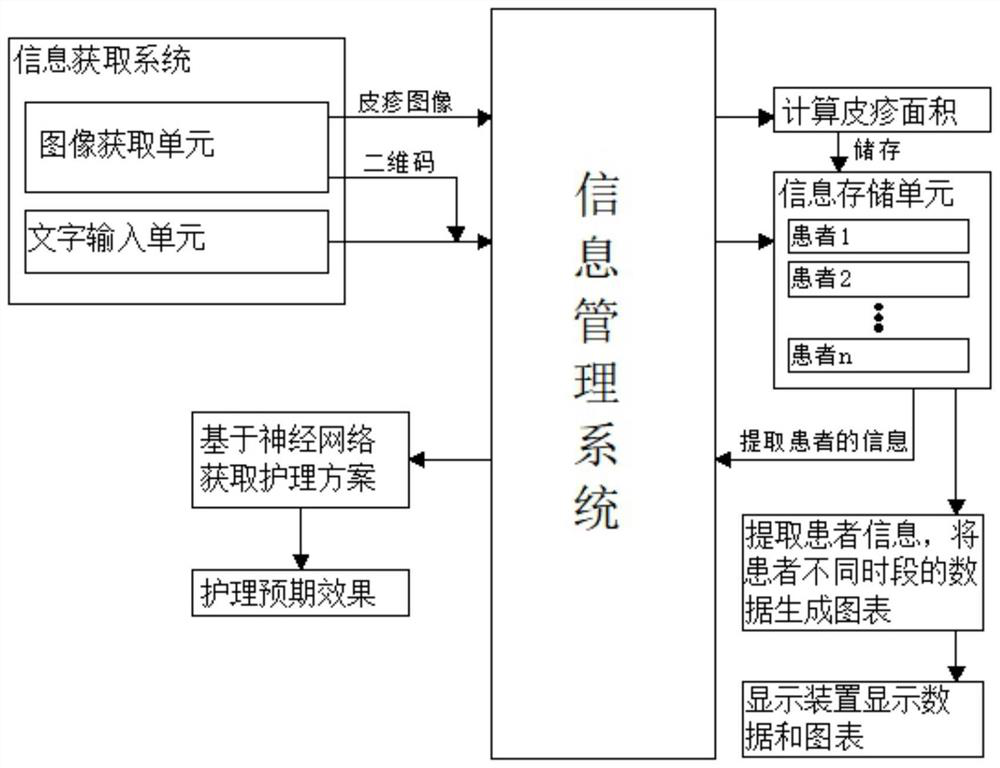
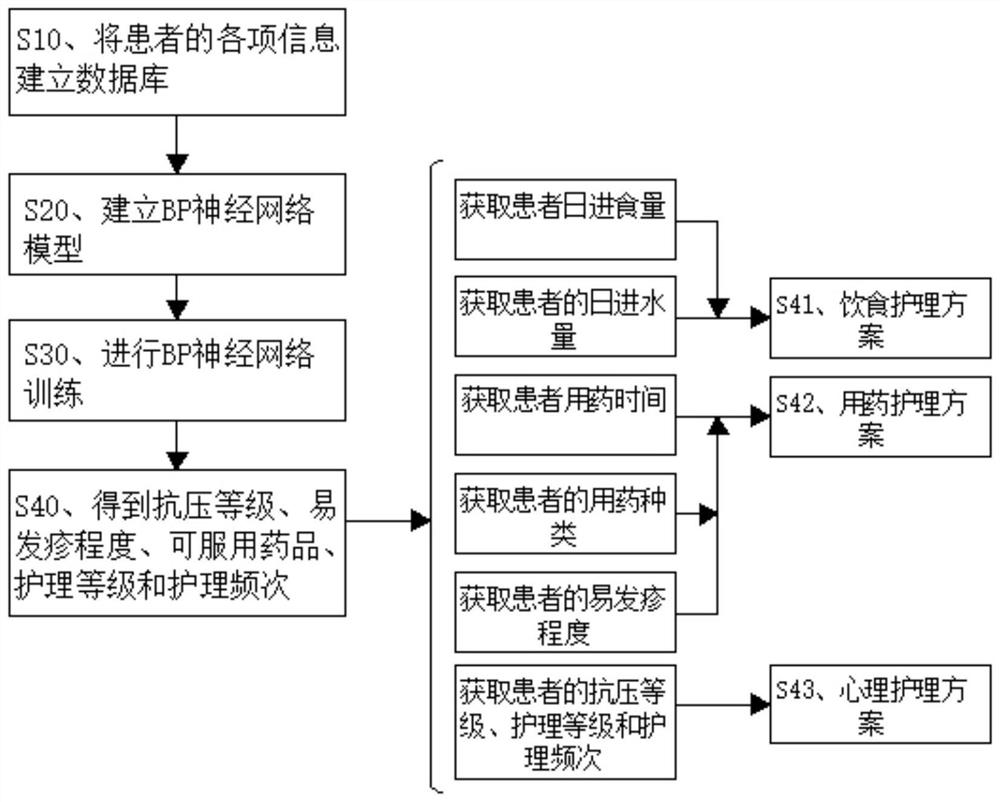
Drug rash patient management system and nursing scheme generation method
PendingCN113066548AEasy to operateConvenient queryPatient-specific dataComputer hardwarePatient management
The invention discloses a drug rash patient management system, and the system comprises an information management system, an information acquisition system, an information storage unit and a display device. The information management system is used for uniformly deploying the information acquisition system, the information storage unit and the display device, and is used for calculating and processing each piece of information; the information acquisition system comprises an image acquisition unit and a character input unit, the character input unit inputs information of a patient, and an image system acquires two-dimensional code or rash image information; and the information storage unit is used for storing various information of the patient. According to the method for generating the nursing scheme for the patient with the drug rash, different nursing schemes are automatically generated, and various information of the patient is considered, so that exclusive nursing schemes can be generated according to different patients, the later nursing effect is better, and the time consumed by automatically generating the nursing schemes is shorter, so that medical staff can obtain a nursing scheme more quickly, and a large amount of time is saved.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
New application of HLA-B* 13 gene
ActiveCN112143787AReduce generationMicrobiological testing/measurementAgainst vector-borne diseasesWhite blood cellPharmaceutical Substances
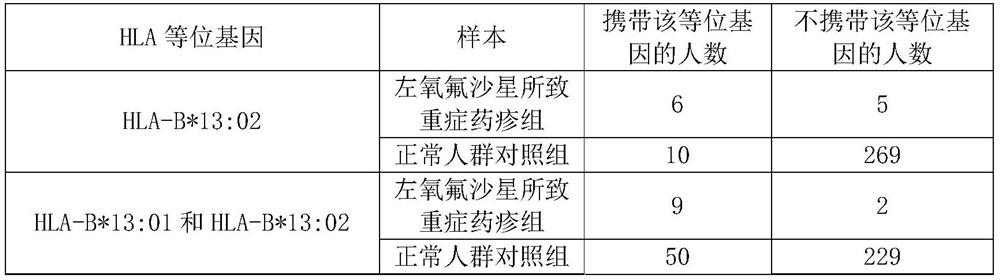
The invention discloses a new application of an HLA-B* 13: 02 allele. The invention discloses an application of a substance for detecting an HLA-B* 13: 02 allele or a substance for detecting HLA-B* 13: 01 allele and the HLA-B* 13: 02 allele in preparation of a product for detecting or evaluating the adverse drug reaction risk of human responding to levofloxacin or the severe drug rash risk causedby levofloxacin. Experiments prove that alleles of human leukocyte antigen genes, namely HLA-B* 13: 01 and / or HLA-B* 13: 02, are related to severe drug rash risks caused by levofloxacin. HLA-B* 13: 01and / or HLA-B* 13: 02 alleles can be used as marker genes for predicting the occurrence risk of severe drug rash caused by levofloxacin.
Owner:FUDAN UNIV +1
Medicine rash plasma exosome protein and kit thereof
PendingCN114162441AReduce generationLow costNon-rotating vibration suppressionRemovable lids/coversDrug rashBlood plasma
The invention provides a medicine rash plasma exosome protein and a kit thereof, and relates to the technical field of kits. According to the kit for the medicine rash plasma exosome protein, a box body and a box cover, a limiting plate is fixedly arranged in the box body, a plurality of containing through grooves are formed in the limiting plate, positioning assemblies are arranged in the containing through grooves, a bearing plate is arranged at the bottom of the limiting plate, and bearing mechanisms are fixedly arranged at the positions, corresponding to the containing through grooves, of the bearing plate; the bearing mechanism comprises a fixing cylinder, a positioning assembly is also arranged in the fixing cylinder, and buffering sets are arranged at the positions, corresponding to the containing through grooves, of the box cover. According to the kit for the medicine rash plasma exosome protein, vibration energy from reagent bottles is absorbed through a plurality of elastic strips in the two positioning assemblies and a buffer assembly arranged at the bottom of the box cover in a matched mode, so that the reagent bottles are stably and neatly located in the box body, and the possibility that reagent liquid in the reagent bottles generates foam due to shaking is reduced.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Application of CCR4 receptor antagonist in preparation of medicine for treating drug rash
ActiveCN110934874APain reliefShort onset timeOrganic active ingredientsDermatological disorderCCR4 ReceptorDrug rash
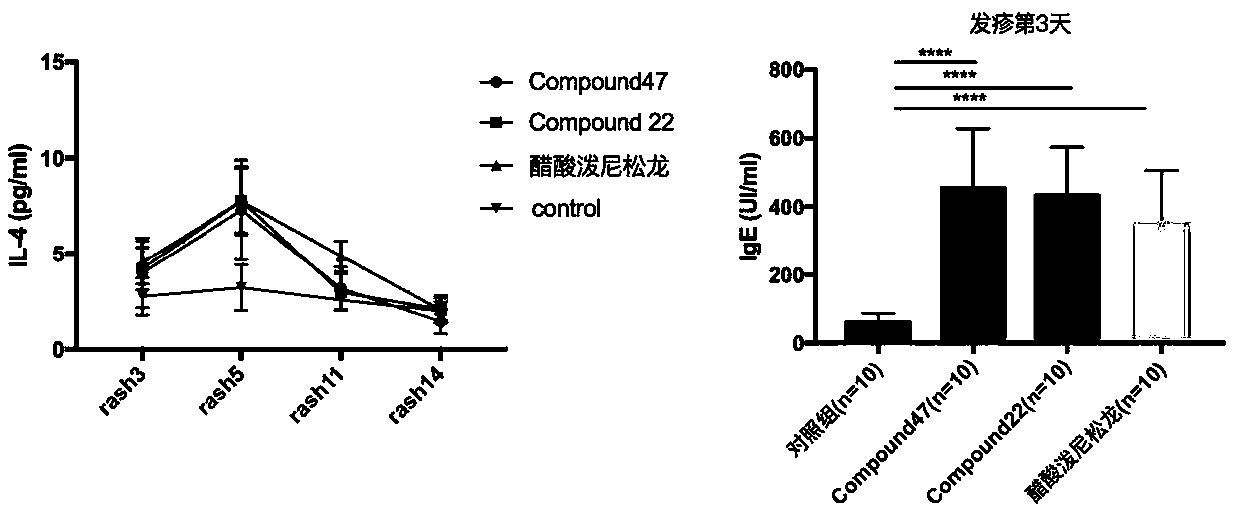
The invention discloses application of a CCR4 receptor antagonist, particularly to application of a CCR4 receptor antagonist in preparation of medicine for treating drug rash. The CCR4 receptor antagonist has the better effect than prednisone acetate and has the quick effect, so that the pain of a patient is relieved more quickly. The CCR4 receptor antagonist has a quick effect especially on the eruption rash or urticaria type rash to alleviate the pain of the patient quickly. When the CCR4 receptor antagonist is AZD2098, the CCR4 receptor antagonist has a good curative effect on the drug rash, especially on the eruption rash; and when the CCR4 receptor antagonist is Compond22, the CCR4 receptor antagonist has a good curative effect on the drug rash, especially the urticaria type drug rash.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
Use of hla-a*02:01 and hla-c*01:02 in drug eruption caused by tanshinone
ActiveCN109609616BReduce generationBlocking interactionMicrobiological testing/measurementAntigenDrug adverse reactions
The invention discloses the use of HLA-A*02:01 and HLA-C*01:02 in drug eruption caused by tanshinone. The invention provides the application of a substance for detecting whether a human has an HLA-A*02:01 allele in the preparation of a product for detecting or evaluating the risk of adverse drug reactions in response to tanshinone in humans. The experiment of the present invention proves that human leukocyte antigen gene—HLA-A*02:01 and / or HLA-C*01:02 allele is related to the onset of drug eruption caused by tanshinone. HLA‑A*02:01 and / or HLA‑C*01:02 alleles can be used as marker genes to predict tanshinone-induced drug eruption.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV +1
Traditional Chinese medicine nursing agent for treating rash caused by hand-foot-and-mouth disease
A traditional Chinese medicine nursing agent for treating rash caused by hand-foot-and-mouth disease is prepared from raw materials in parts by weight as follows: 5-9 parts of amur cork-tree, 7-12 parts of purslane herb, 4-9 parts of grassleaf sweetflag rhizome, 15-20 parts of Tokyo violet herb, 3-4 parts of natural indigo, 5-8 parts of largeleaf gentian root, 12-15 parts of kudzuvine root, 10-16 parts of honeysuckle flower, 3-8 parts of creeping euphorbia, 5-12 parts of dandelion, 12-17 parts of common bombax flower, 4-6 parts of mint, 8-12 parts of honeysuckle stem, 3-4 parts of dwarf lilyturf tuber, 12-15 parts of fragrant solomonseal rhizome, 10-15 parts of divaricate saposhnikovia root, 8-12 parts of beautiful sweetgum fruit, 2-5 parts of Chinese gall, 3-7 parts of cape jasmine fruit, 11-14 parts of smoked plum, 9-15 parts of officinal magnolia bark, 3-6 parts of tribulus terrestris fruit, 8-13 parts of atractylodes rhizome, 3-5 parts of dendrobium stem, 1-3 parts of Gromwell root, 5-10 parts of climbing groundsel herb and 5-10 parts of common cnidium fruit.
Owner:QINGDAO MUNICIPAL HOSPITAL
Externally applied medicine for treating eczematous dermatitis as well as preparation method and application thereof
InactiveCN112791138AEasy to prepareNo side effectsPharmaceutical delivery mechanismImmunological disordersDiseaseFormulary
The invention relates to the field of medicines, and particularly discloses an externally applied medicine for treating eczematous dermatitis and a preparation method and application thereof. Raw materials of the medicine comprise lithospermum, divaricate saposhnikovia root, Chinese angelica, radix angelicae, radix rehmanniae recens, honeysuckle buds and flowers and scutellaria baicalensis. The externally applied medicine for treating eczematous dermatitis is effective on eczematous dermatitis, has effects on related diseases such as drug rash, neurodermatitis and mosquito bite swelling, and can be directly smeared on an affected part due to the adoption of a pure traditional Chinese medicine formula. The medicine is free of side effects caused by hormone ointment such as hyperpigmentation, coarse pores and induction of other diseases, has the advantages of being easy to prepare, convenient to use, stable in medicine effect, safe and free of stimulation. The preparation method is simple, and the medicine is free of side effects, and solves the problems that most of existing medicines for treating eczema are western medicines which has many side effects, and the medicine has a wide market prospect.
Owner:浙江中医药大学附属第二医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com