Application of fraxinellone in preparation of drugs for treating allergic skin diseases
A technology for allergies and skin diseases, applied in allergic diseases, skin diseases, drug combinations, etc., can solve the problems of patients with ineffectiveness, easy recurrence, and many adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
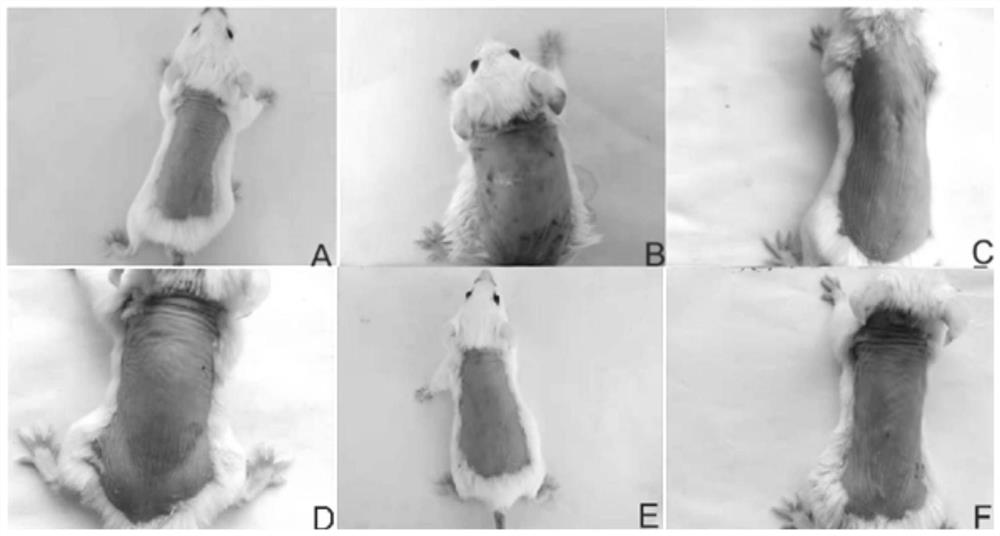
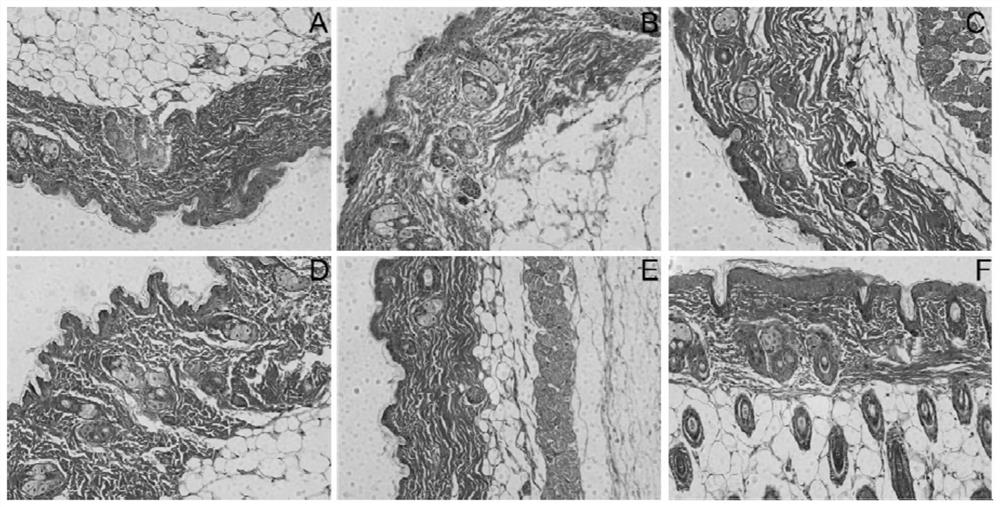
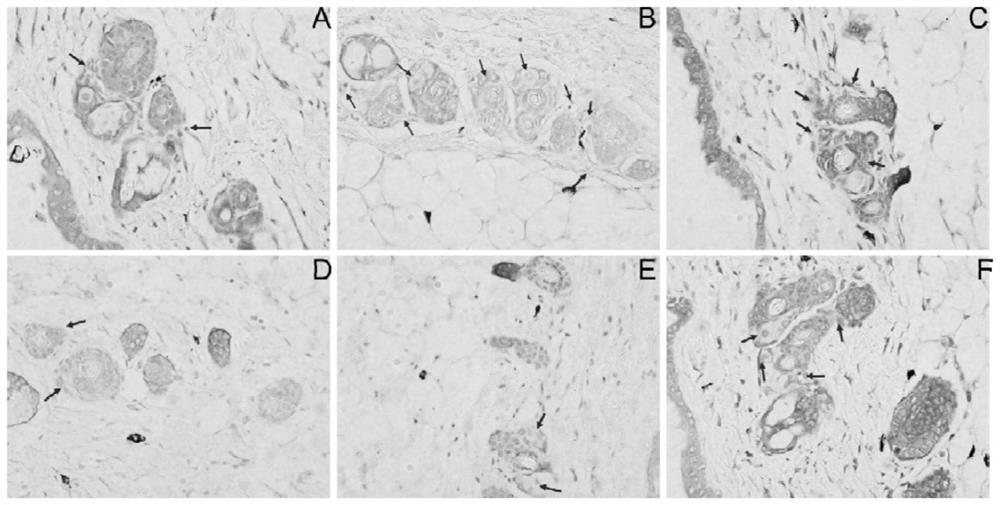
[0026] Passive skin allergy test (PCA)
[0027] Sensitization: SD rats, weighing 200-250 g, both male and female, provided by the Guangdong Experimental Animal Center. Take 10 SD rats, and inject 1 mL of physiological saline solution containing 1 mg ovalbumin and 10 mg aluminum hydroxide intraperitoneally into each SD rat for immunization; once every other day, a total of 3 times, they were killed 12 days after the last sensitization, and the abdominal aorta Blood collection, 3000r·min -1 Centrifuge for 10 min, take the supernatant, prepare antiserum, and store it at -20°C for future use.
[0028] Passive sensitization: BALB / c mice, half male and half male, 18-22 g, SPF grade, purchased from Zhejiang Weitong Lihua Experimental Animal Technology Co., Ltd. Sixty Balb / c mice were randomly divided into normal group, model group, loratadine group, low-dose alkone group, middle-dose alkone group, and high-dose alkone group.
[0029] The low-, medium-, and high-dose groups were ad...
Embodiment 2
[0062] Tablets containing arketone
[0063] Formula: alkone 200g, lactose 88g, hydroxymethyl starch sodium 9g, magnesium stearate 3g.
[0064] Preparation method: take the alkone raw material drug, lactose and sodium hydroxymethyl starch (75% of the formula amount) according to the formula and mix them, pass through a 120-mesh sieve for 3 times, and mix well. Add an appropriate amount of binder to make a soft material. The soft material is passed through a 18-mesh sieve, and the wet granules are dried in an oven at 50°C for 1 hour. Formula quantity), mix well, compress into tablets, and get ready.
Embodiment 3
[0066] Capsules containing arketone
[0067] Recipe: alkone 180g, starch 50g, dextrin 50g.
[0068] Preparation method: Weigh alkone raw material, add starch and dextrin, mix well, use 90% ethanol as wetting agent for wet granulation, vacuum dry at 50°C and then granulate, pass through 40-mesh sieve and 200-mesh sieve The fine powder is removed, and the medicinal powder granules are packed into No. 3 capsules in an environment with a humidity lower than 60%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com