Application of HLA-C*02:01:01 alleles in detection or evaluation of adverse drug reactions of efavirenz
A technology of HLA-C and alleles, applied in the field of biomedicine, to reduce the occurrence of severe drug eruption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
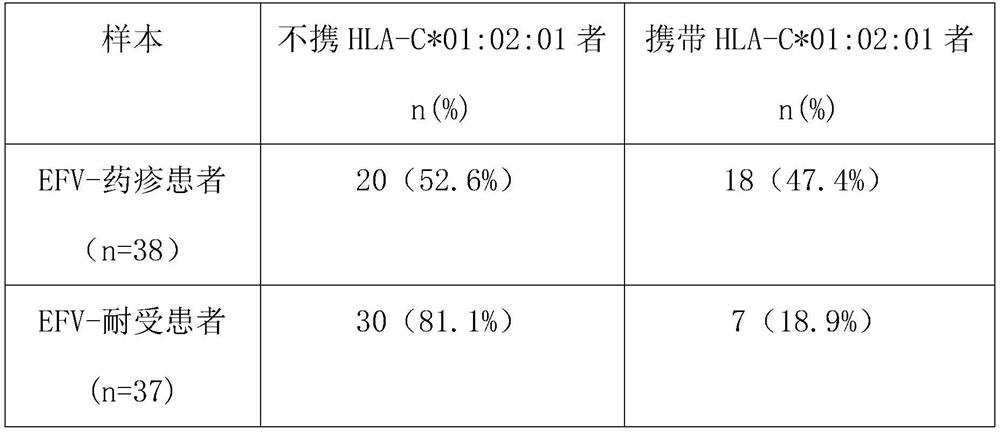
[0033] Example 1 The HLA-C*01:02:01 allele is a risk allele marker for drug eruption caused by efavirenz.
[0034] 1. Sample
[0035] 1. Case samples (drug eruption group caused by efavirenz)
[0036]The case samples were 38 patients with drug eruption caused by efavirenz in Yunnan Province, China. The 38 patients received ART initial treatment for HIV / AIDs. The ratio of male to female in patients was 0.9:1, and the average age was 39 years old. The time from the start of taking EFV to the appearance of rash symptoms was 8-26 days (average 14 days). All patients stopped using efavirenz due to drug eruption caused by efavirenz, and no drug eruption occurred after stopping and changing EFV treatment. The diagnosis and classification of drug eruption are based on laboratory examination, medical history, incubation period, and the shape and distribution of rash. Drug eruptions caused by any other drug and other diseases with similar symptoms were excluded.
[0037] 2. Contro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com