Human leukocyte antigen gene detection kit for screening skin adverse reactions caused by clindamycin
An adverse reaction and kit technology, which is applied in biological testing, microbial determination/inspection, measurement devices, etc., and can solve problems such as no kit reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Collection and extraction of genes
[0093] Patients with drug eruption caused by clindamycin came from Huashan Hospital Affiliated to Fudan University in Shanghai, China. They participated in the research of this subject under the premise of informed consent, collected blood, and signed an informed consent form. All enrolled patients were excluded from other diagnoses such as viral eruption and toxic erythema through medical history and skin pathological examination. All patients took clindamycin intravenously or orally within 2 months before the onset of rash. There are two groups of controls in this experiment. One is 283 healthy people from the human MHC database (dbMHC). In the lindamycin-resistant group, all patients received clindamycin intravenously for more than 2 months without any drug reaction;
[0094] Take 300 ul of sample blood, extract DNA according to the kit instructions, and detect the DNA concentration and purity with an ultraviolet spec...
Embodiment 2
[0095] Example 2: Detection of HLA genotyping
[0096] The present invention adopts PCR-SSO method (recommended Kit-One Lambda, CA, USA) for HLA genotyping. Its principle is to amplify the polymorphic region of HLA first, carry out isotope or non-isotope labeling on the PCR products during the amplification process, and then design a series of oligonucleotide probes for the PCR amplification products according to the principle of base pairing and immobilize them in Finally, the PCR product was hybridized with the probe on the membrane, and autoradiography was used to judge the result according to the signal. HLA genotyping was performed according to the standard steps of the kit. (i.e. DNA samples, substrates, Taq enzymes, and primers are mixed and evenly mixed, added to the amplification plate, amplified according to the conditions in the kit instructions, and the amplified products are hybridized, stained, and plate read). Results were analyzed by HLA Fusion software (On...
Embodiment 3
[0097] Example 3: HLA-B*51:01 is related to drug eruption caused by clindamycin
[0098] Statistics: SPSS20.0 was used to calculate Odds Ratio (OR) and its 95% confidence interval, P value was corrected by Bonferroni, Fisher's exact test was used for statistical analysis, and the statistical significance level was set at P less than 0.05.
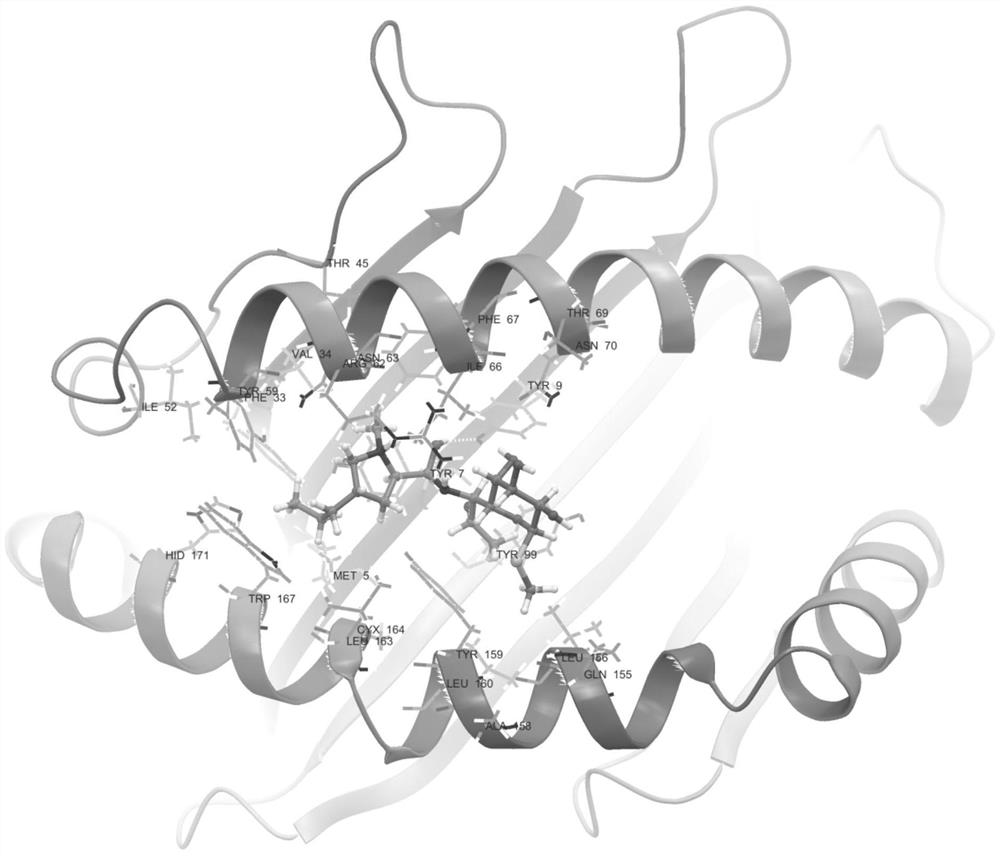
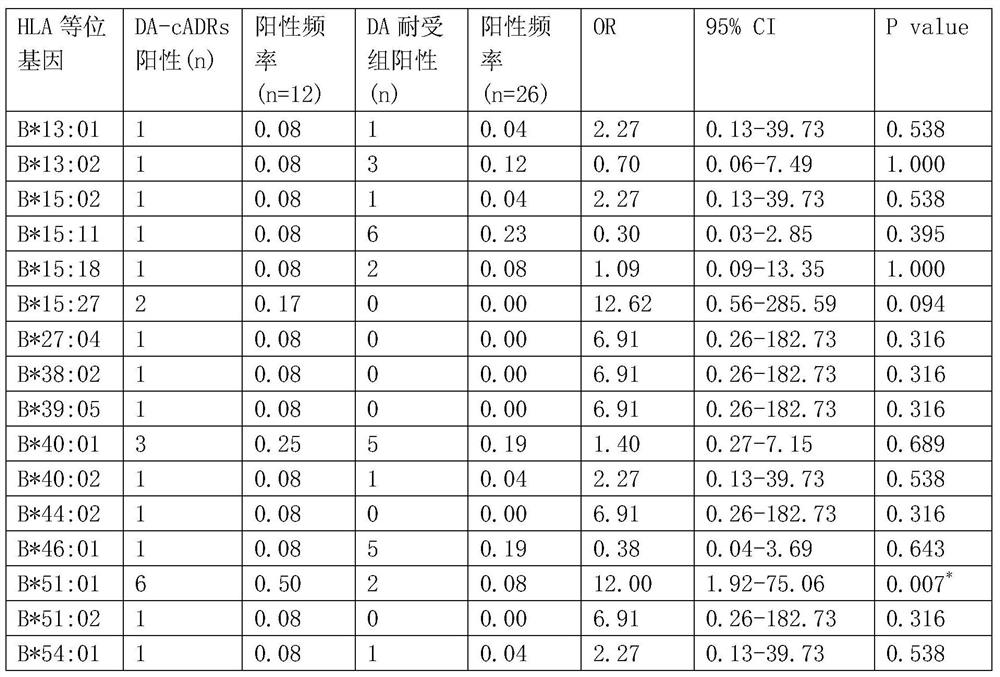
[0099] The results showed that: 12 patients with clindamycin-induced drug eruption were collected, and 26 cases were in the clindamycin-resistant group. There were statistically significant differences between the clindamycin-resistant group (as shown in table 1) and the clindamycin-resistant group (as shown in table 2); the binding model of HLA-B*51:01 and clindamycin figure 1 Shown (model diagram of clindamycin binding to sHLA-B*51:01).
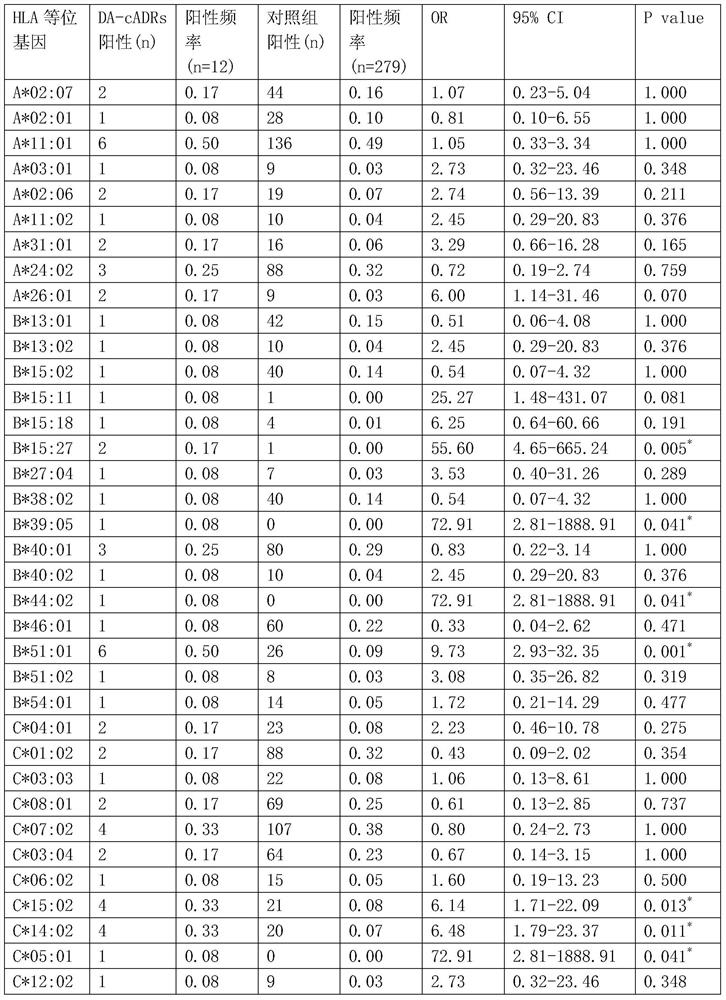
[0100] Table 1. Positive ratio of HLA-B*51:01 in the drug eruption group caused by clindamycin and the normal control group
[0101]
[0102] *The difference is statistically significant (P<0.05). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com