Application of spd_0310 protein as a target in the preparation of drugs for preventing and treating Streptococcus pneumoniae infection
A Streptococcus pneumoniae and drug technology, applied in the field of protein science, can solve the problems of low detection sensitivity, low expression level, and undetectability, and achieve high conservation, strong toxicity, and good broad-spectrum effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The specific combination of embodiment 1SPD_0310 protein and hemin
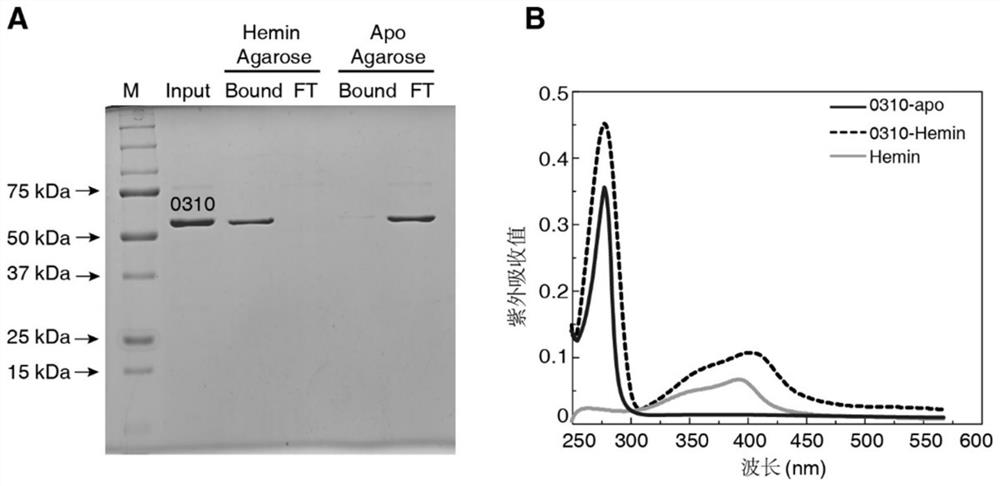
[0071] We successively explored the SPD_0310 protein and Fe 3+ , heme (hemin, purchased from Sigma-Aldrich), fch (iron pigment, purchased from Sigma-Aldrich) and Mn 2+ The binding status of the protein, that is, the protein was incubated with the above five ligand small molecules for 6 hours, and then the chelated PBS buffer was used for overnight dialysis treatment for 3 times. Finally, we found that other metals failed to bind through ICP-MS detection. For 0310 protein, only 0310-hemin has an iron content detection value of 7.524 mg / L, which is 134 μM, while the protein concentration is 30 μM. It can be seen that the binding ratio of SPD_0310 protein to hemin is about 1:4, which indicates that hemin may be the binding substrate of the protein.
[0072] In order to further verify this inference, we first incubated 4 μg of 0310 protein with hemin-Agarose and apo-Agarose magnetic beads (purchased from...
Embodiment 2
[0074] Example 2 Surface plasmon resonance (SPR) detects the binding of SPD_0310 protein to hemin
[0075] Utilize the Open-SPR instrument (premedi, nicoya, Canada) to detect the combination of protein and ligand hemin, with 1 × PBS buffer as mobile phase buffer, with 0.2M N-hydroxysuccinimide (EDC) and The 50mM N-ethyl-N'-(diethylaminopropyl)-carbodiimide (NHS) 1:1 (volume ratio) mixed solution will be purchased for the nano-gold-COOH sensor chip (premedi, nicoya, Canada) surface amino group activation, diluted with 10nM sodium acetate buffer solution of pH 4.5 to a final concentration of about 50 μg / mL, injected at a flow rate of 30 μL / min for 500 s, and then injected the diluted SPD_0310 protein solution with 1M ethanolamine pH 8.5 The blocking solution was injected for 7 minutes to block the activated chip surface, that is, the SPD_0310 protein was tightly combined with the amino group of the chip through the mobile phase. Dilute hemin with sample diluent to different con...
Embodiment 3
[0077] CD spectrum detection of embodiment 3SPD_0310 protein binding to Hemin
[0078] The detection results of the above SPR experiments suggest that the main biological function of the SPD_0310 protein is the binding and transport of hemin. So, will the 0310 protein cause a change in protein conformation after binding the ligand? To this end, we used circular dichroism chromatography to detect the secondary structure (secondary-structure) of Apo-0310 and 0310-hemin respectively [5] .
[0079] Such as Figure 5 As shown, the CD peak patterns of Apo-0310 protein and 0310-hemin are almost the same. After detecting the content of various indicators of the secondary structure by CDPro software, it is found that there is no obvious difference between the two, which shows that the binding of the protein to hemin will not cause drastic changes in its secondary structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com