New application of HLA-B* 13 gene
A kind of HLA-B, allele technology, applied in the new use of HLA-B*13:02 gene, HLA-B*13:01 gene field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
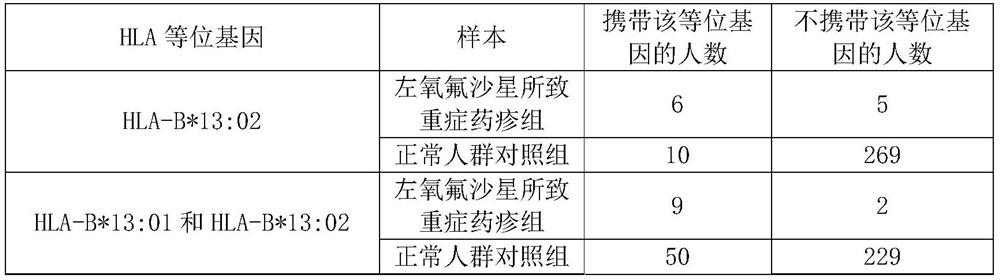
[0059] Example 1, HLA-B*13:01 and / or HLA-B*13:02 alleles are genetic markers of risk of severe drug eruption caused by levofloxacin
[0060] 1. Sample
[0061] 1. Case samples (severe drug eruption group caused by levofloxacin)
[0062] The case samples in this example were 11 patients with severe drug eruption caused by levofloxacin from Huashan Hospital Affiliated to Fudan University in Shanghai, China. These 11 patients received levofloxacin for pulmonary infection, urinary tract infection or other infections. Among the 11 patients, there were 8 males and 3 females, with an average age of 47 years. The time from the start of levofloxacin to the appearance of severe drug eruption symptoms ranged from 1 day to 21 days (average 7.9 days). The 11 patients included 4 SJS, 4 TEN, 1 SJS / TEN overlap, 1 DRESS, and 1 AGEP. The diagnosis of severe drug eruption is based on the patient's medical history, clinical manifestations and laboratory tests.
[0063] 2. Normal control samp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com