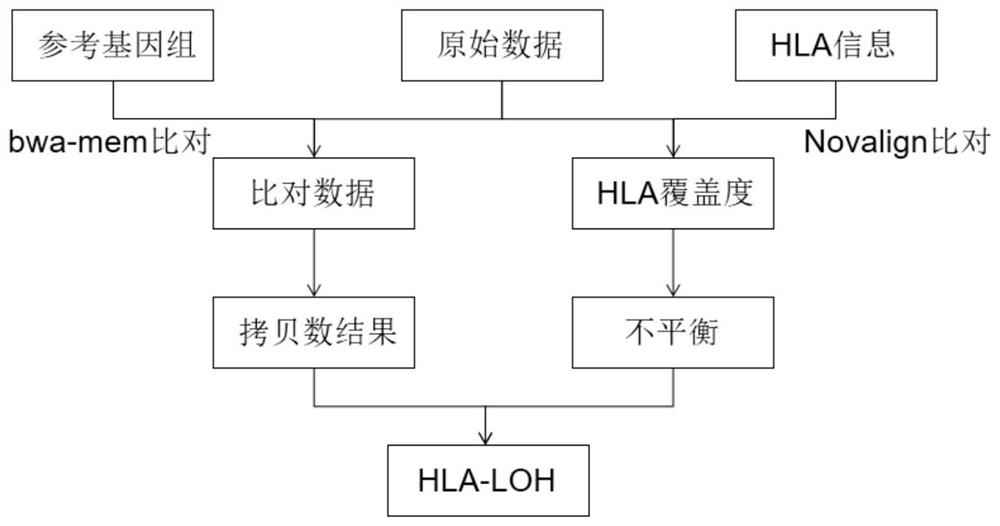
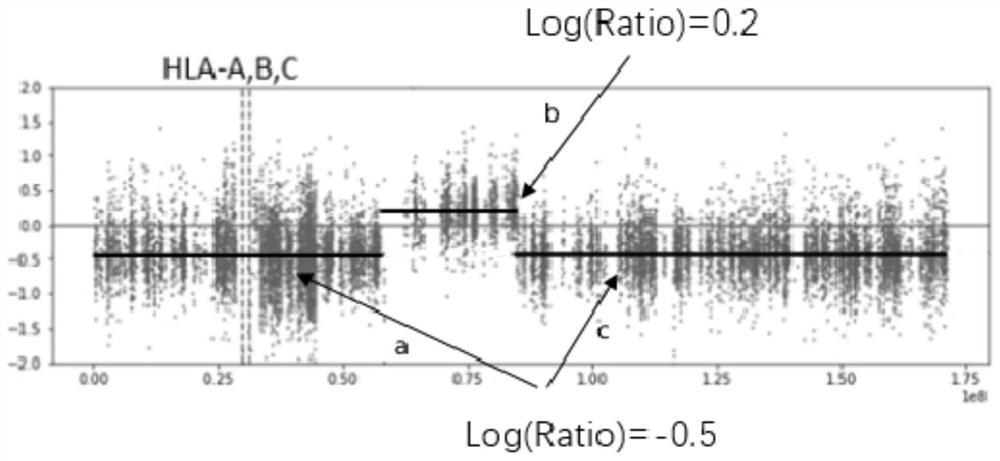
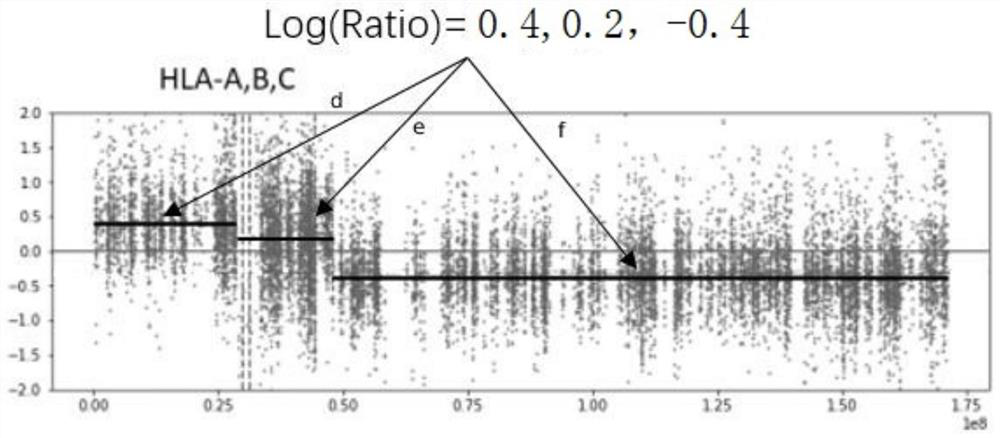
Method and system for detecting HLA heterozygosity deficiency
A technology for loss of heterozygosity and balance detection, applied in the field of bioinformatics, can solve problems such as HLA gene deletion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] In this example, the samples used were taken from patients with nasopharyngeal and throat cancer, the tumor samples were taken from cancer tissues, and the control samples were taken from the blood leukocytes of the same patient, specifically the samples from patients with high TMB (tumor mutation burden) who were ineffective in immunotherapy T17121390189- KY438-VS-B17120989463-KY438 (denoted as sample_1_1) and patient sample F17120989201-KY438-VS-B17120989370-KY438 (denoted as sample_1_2) from patients with effective immunotherapy and high TMB.
[0129] The specific steps for the detection of sample T17121390189-VS-B17120989463-KY438 in this example are as follows:
[0130] 1. The Agilent WES SureSelect Human All Exon V6 method was used for sequencing, and the sequencing platform was Illumina NextSeq 2000.
[0131] The Agilent WES SureSelect Human All Exon V6 method refers to the following website:
[0132] https: / / www.agilent.com / cs / library / datasheets / public / SureSele...
Embodiment 2
[0149] The method involved in this embodiment and the software version used are the same as those in Embodiment 1.
[0150] The sample number of this embodiment is DN1902862AZZAA02-VS-DN1902862XYZAA02 (denoted as sample_2_1), which was taken from a patient with nasopharyngeal and throat cancer, the tumor sample was taken from cancer tissue, and the control sample was taken from blood leukocytes of the same patient.
[0151] The sample test results of this embodiment are as follows:
[0152] 1. Obtain the copy number variation result of the sample, and there is no copy number deletion in the HLA region.
[0153] 2. Here, the p-value of the HLA-A gene is equal to 2.27E-04, which is less than the threshold value of 0.001, indicating that there is an imbalance between the two genotypes of the HLA-A gene.
[0154] 3. Combine the above two results to judge the HLA allele amplification / deletion status, and judge it as non-HLA-LOH.
[0155] Table 2 below is the information of the sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com