Oligopeptide-free cell culture media
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of BAV-Medium
[0072] Oligopeptide-free medium (BAV-medium) was prepared with basal DMEM / HAM's F12 (1:1) comprising inorganic salts, amino acids, vitamins and other components (Life technologies, 32500 Powder). Also added were L-glutamine (600 mg / L), ascorbic acid (20 μM), ethanol amine (25 μM), Synperonic® (SERVA) (0.25 g / L), sodium selenite (50 nM). Additionally essential amino acids were supplemented to the cell culture medium: L-Asparagine.H2O 20 mg / L, L-Cysteine.HCl.H2O 15 mg / L, L-Cystine.2 HCl 20 mg / L, L-Proline 35 mg / L, L-Tryptophan 20 mg / L.
example 2
Determination of Cell Counts
[0073] Cell counts from suspension cells or immobilized cells were determined either by counting with a CASY® cell counter as described by Schärfe et al., Biotechnologie in LaborPraxis 10: 1096-1103 (1988), or by citric acid extraction and fluorescent staining of the nuclei followed by counting with a NucleoCounter® (Chemometec, DK). The specific growth rate (μ) is calculated from the increase of the cell densities (Xt) and / or the dilution rate (D) of the steady state of chemostat cultures of suspensions cells over a certain time interval (t):
μ=D+In(Xt / X0) / t
example 3
Determination of FVIII Activity
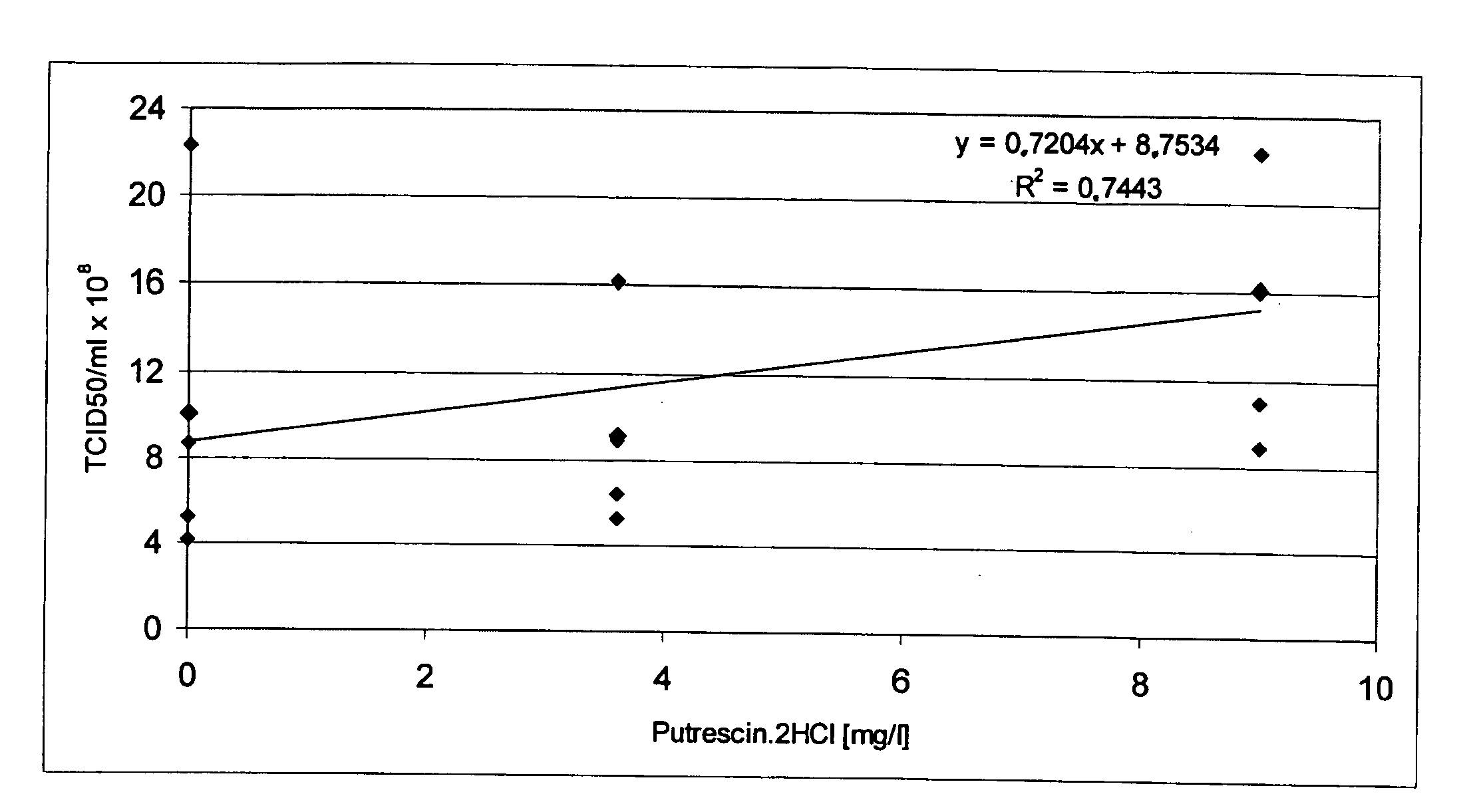
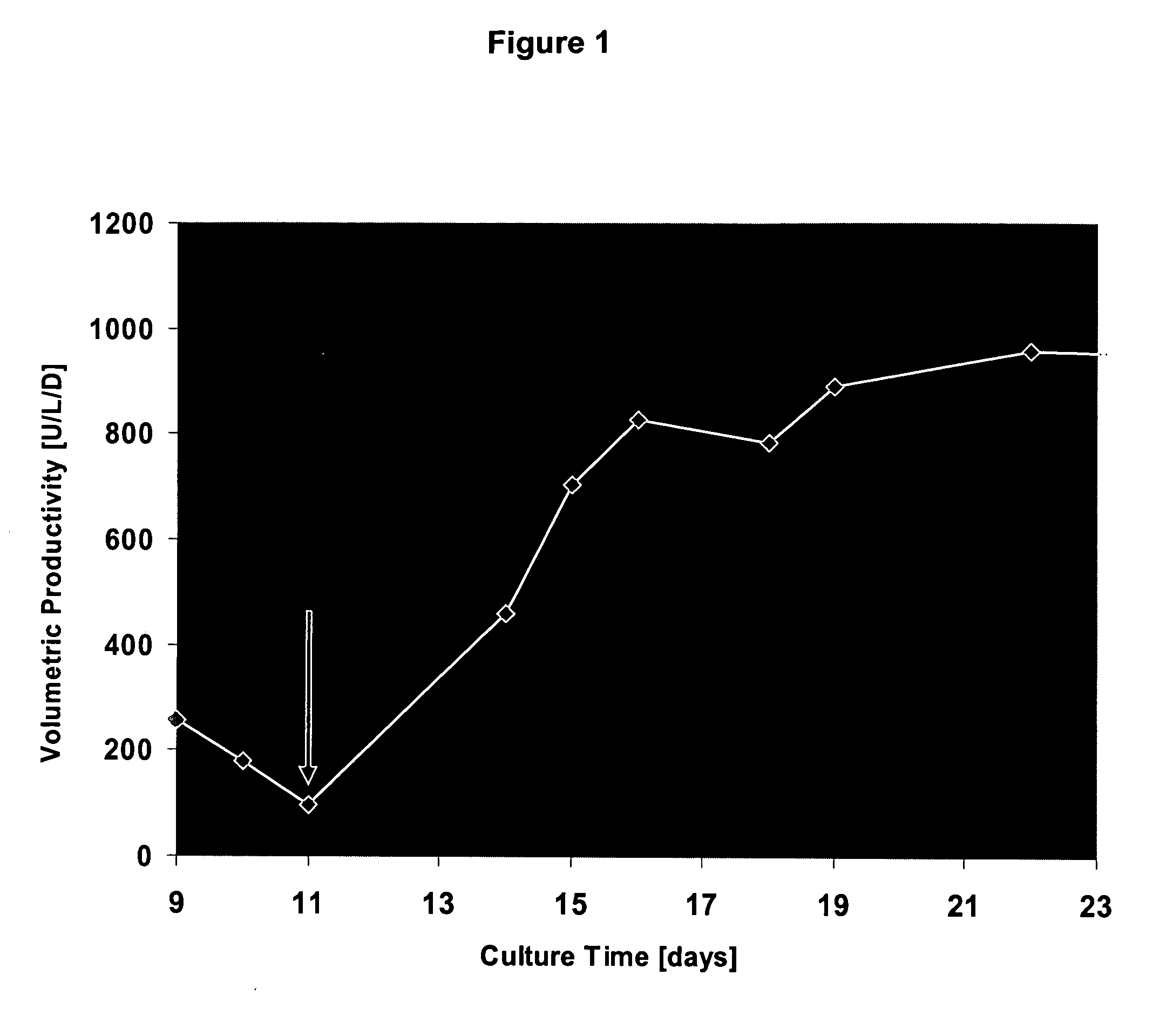
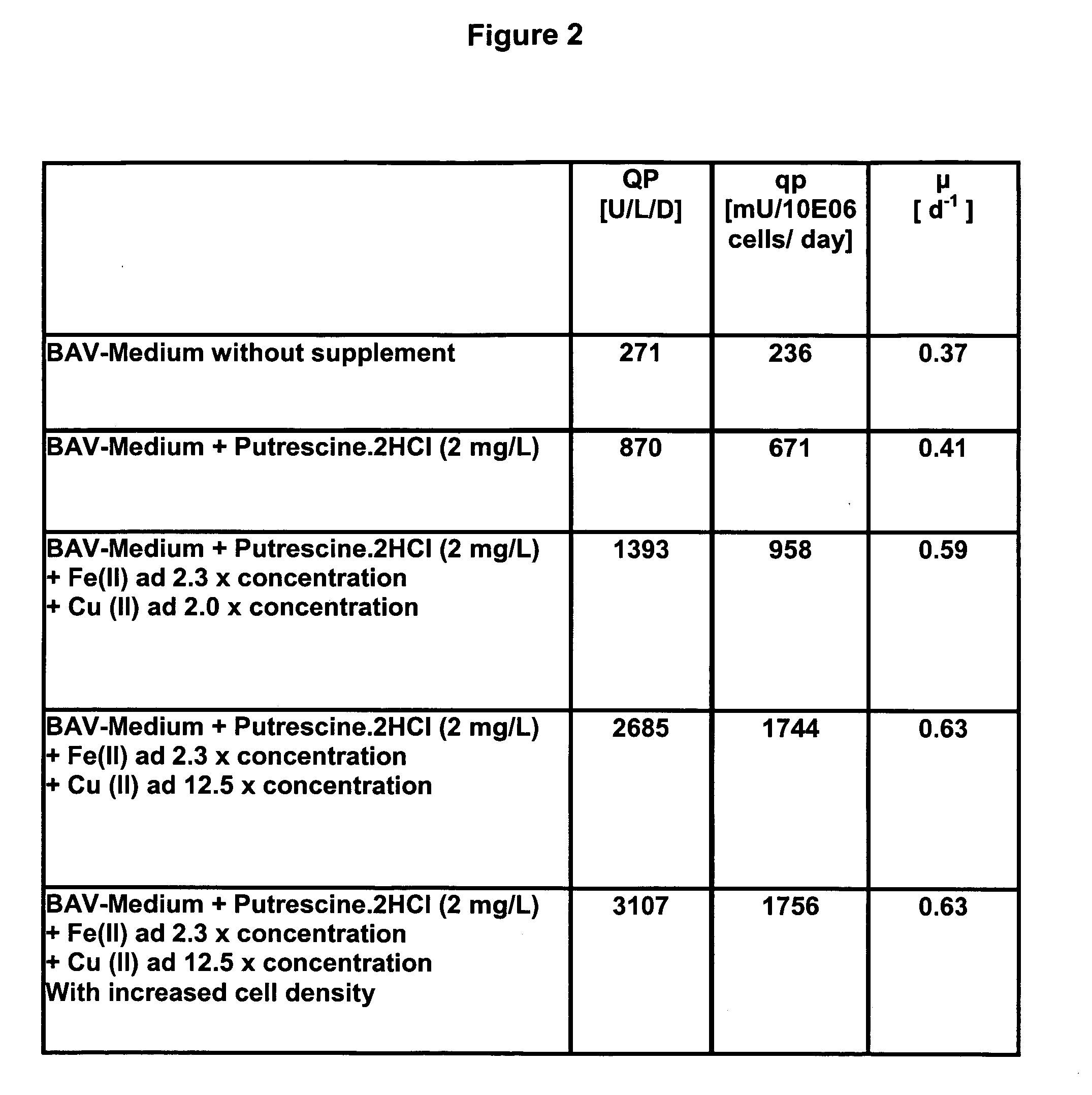
[0074] The activity of Factor VIII (FVIII) (cf. FIGS. 1 to 5) was measured by a chromogenic assay (Chromogenic, Sweden).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com