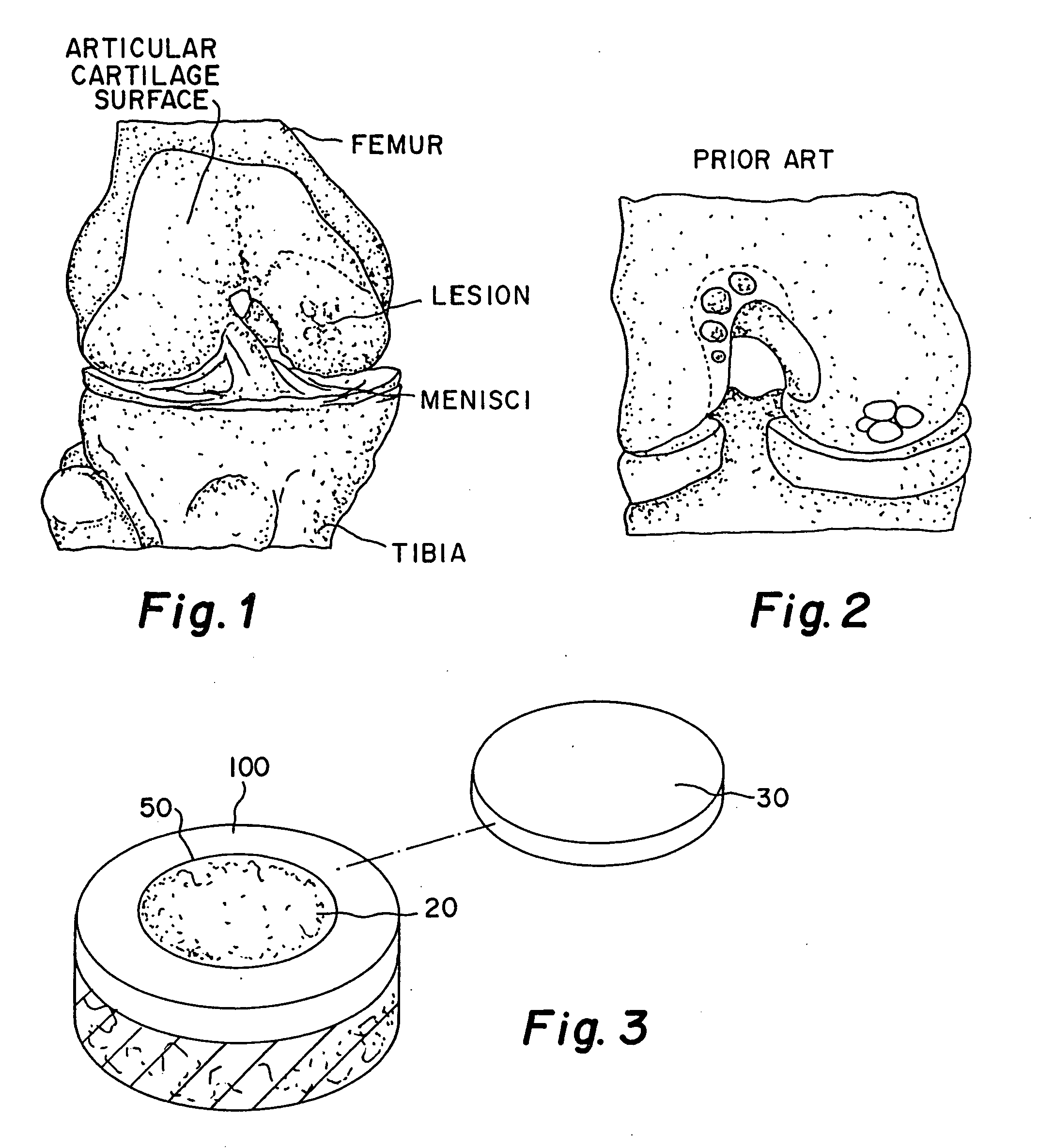
Cartilage repair mixture containing allograft chondrocytes
a cartilage repair and allograft technology, applied in the field of implants, can solve the problems of affecting the healing effect of hyaline cartilage, and affecting the healing effect of articular cartilage lesions, so as to increase the migration and proliferation of chondrocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0037] A matrix of minced cartilage putty consisting of minced or milled allograft articular cartilage which has been lyophilized so that its water content ranges from 0.1% to 8.0% with a cartilage content ranging from 25% to 50% by weight is mixed with a carrier of sodium hyaluronate solution (HA) (molecular weight ranging from 7.0×105 to 1.2×106) or any other bioabsorbable carrier such as hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran, or polymers, the carrier ranging from 50% to 75% by weight. The cartilage is milled to a size ranging from 0.01 mm to 1 mm. In gel form, the minced cartilage which has been lyophilized so that its water content ranges from 0.1% to 8.0% ranging from 15% to 30% by weight and the carrier ranges from 70% to 85% by weight. The particle size of the cartilage when milled is less than or equal to 1 mm dry in the previously stated range. The cartilage pieces can be processed to varying particle sizes and the...
example 2
[0038] A matrix of minced cartilage putty consisting of minced or milled allograft cartilage taken from the same human donor as the chondrocytes noted below which has been lyophilized so that its water content ranges from 0.1% to 8.0% ranging from 25% to 50% by weight is mixed with a carrier of sodium hyaluronate solution (HA) (7.0×105 to 1.2×106)or any other bioabsorbable carrier such as hyaluronic acid and its derivatives, gelatin, collagen, chitosan, alginate, buffered PBS, Dextran, or polymers ranging from 50% to 75% by weight. In a gel form, the minced cartilage which has been lyophilized so that its water content ranges from 0.01% to 8.0% ranging from 15% to 30% by weight and the carrier ranges from 70% to 85% by weight The particle size of the cartilage is less than or equal to 1 mm dry ranging from 0.01 mm to 1 mm. The cartilage pieces can be processed to varying particle sizes and the HA or carrier can have different viscosities depending on the desired consistency of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com