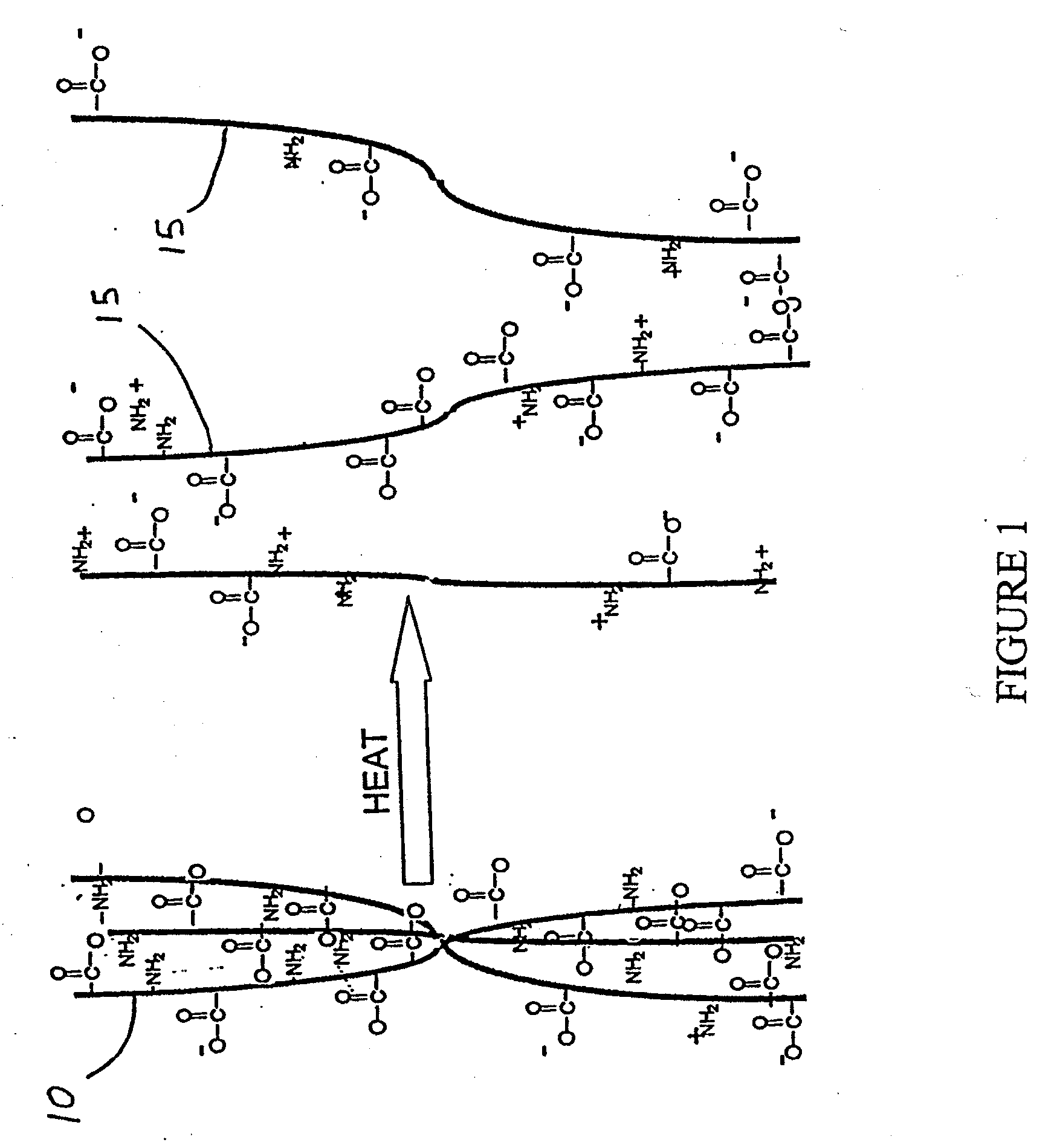
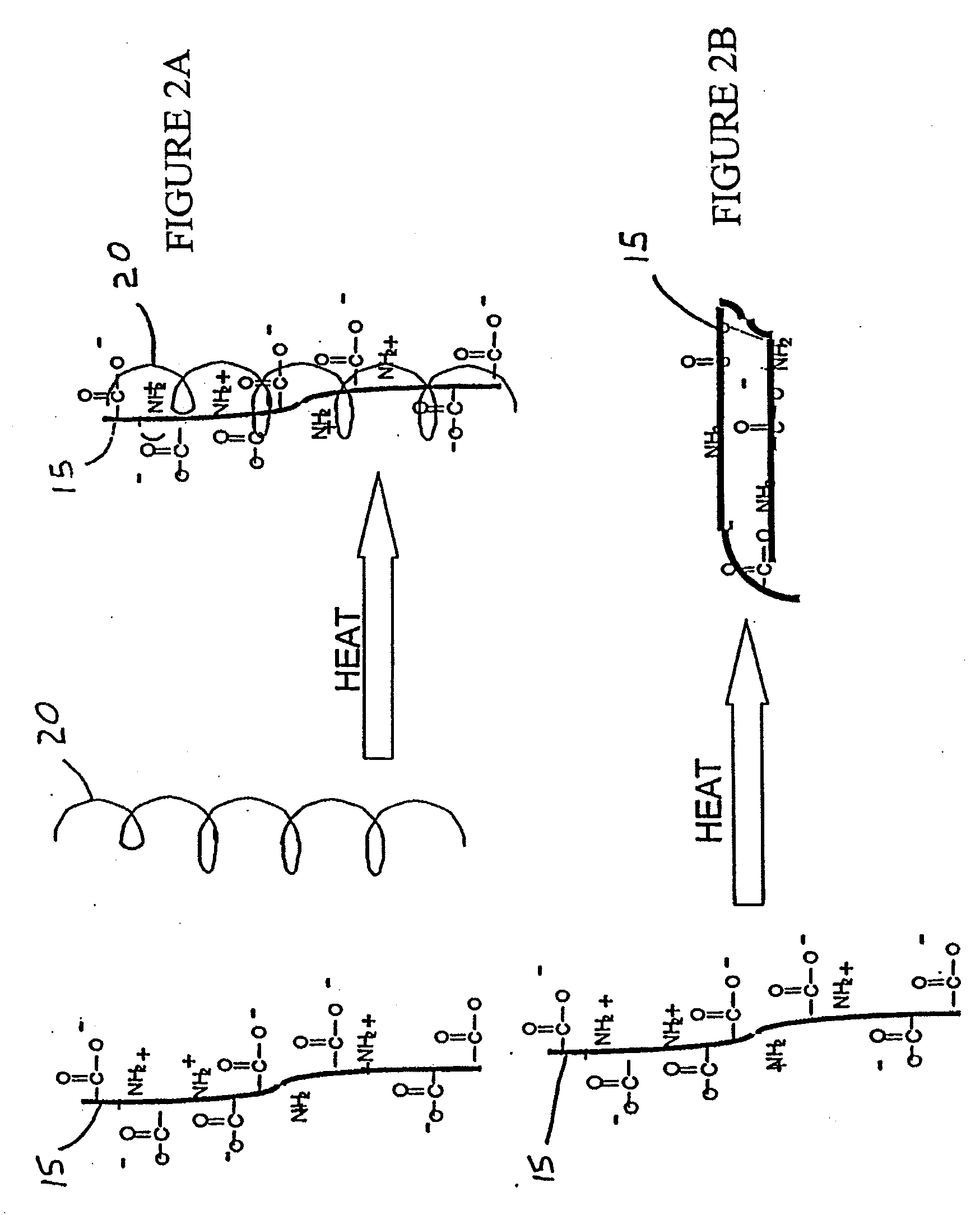
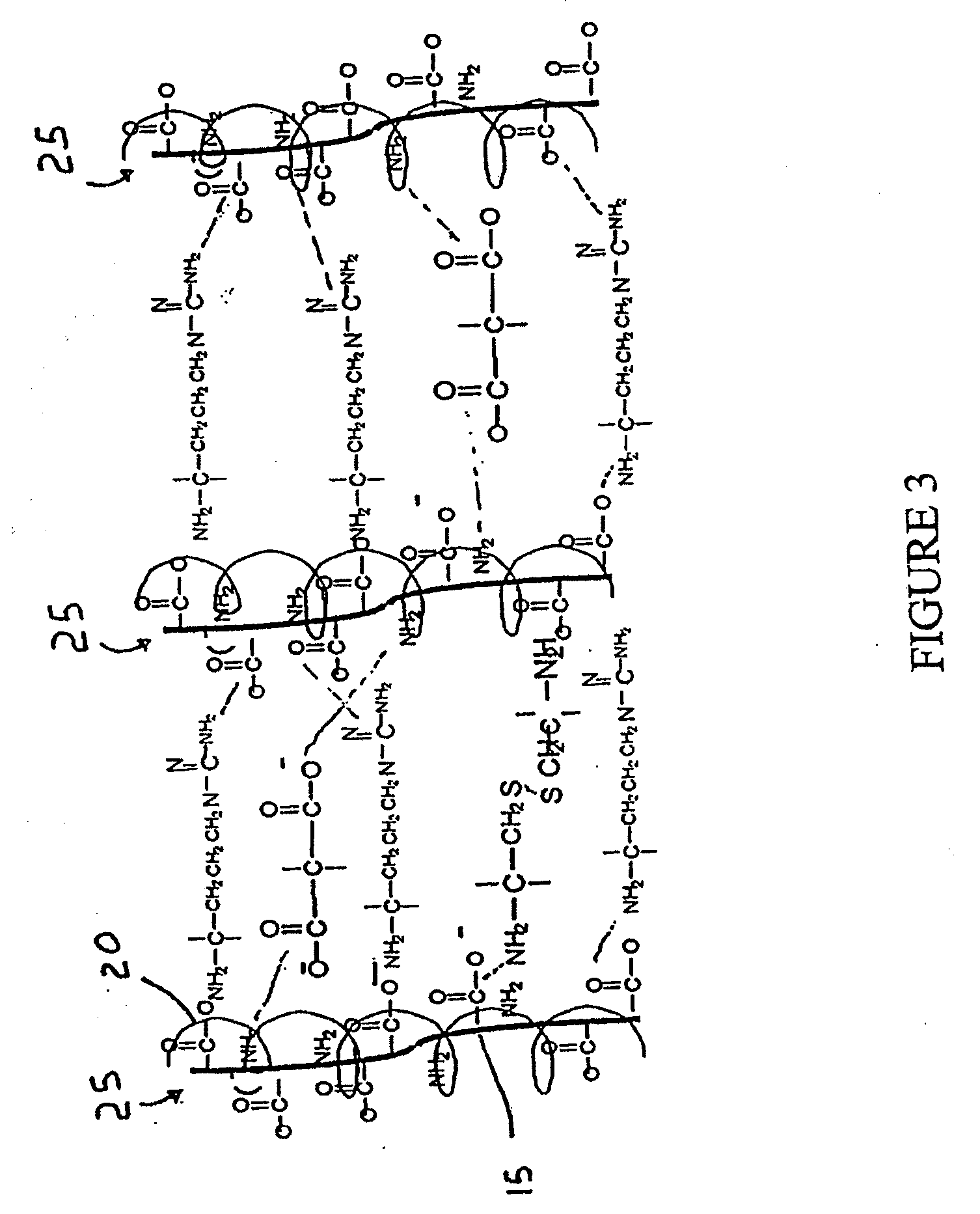
Methods and Compositions for Regenerating Connective Tissue
a technology of connective tissue and composition, applied in the field of methods and compositions for regenerating connective tissue, can solve the problems of large treatment challenges, complete loss of bone tissue across an extended length of bone, and cavitation of bone, so as to facilitate the integration of artificial joints into surrounding tissue, the effect of halting progression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Matrix Preparation
[0139]In one embodiment, the bioactive hydrogel matrix was compounded to yield a final formulation as described above in Table 1. Modified Medium 199 (2.282 L) was placed into a stirred beaker. To the beaker were added L-cysteine, L-glutamic acid, L-lysine, L-alanyl-L-glutamine, and EDTA. While stirring, the solution was heated to 50° C. Next, dextran was added, followed by the addition of gelatin. NaOH (10%) was used to adjust the pH of the matrix solution to a final pH of 7.50±0.05. Finally, additional L-glutamic acid, L-arginine, and L-cysteine were added followed by the addition of zinc sulfate. The amounts of each component used were the amounts necessary to bring the final concentration of each component to the preferred concentration provided in Table 1.
example 2
Effect of Bioactive Hydrogel Matrix on Critical Size Defect in Bone
[0140]The in vivo effect of the matrix on bone repair was examined using the critical size defect model in the rabbit ulna. A defect in a rabbit ulna was created in which the length of the defect was purposefully made to be three times the diameter of the bone, i.e., a 15 mm defect was created in each ulna (the diameter of the bone was approximately 4 mm). It is well documented in the literature that defects of this size will not spontaneously heal (i.e., a critical size defect). Seven rabbits had 15 mm defects surgically created in the ulna of each forelimb. Further, the periosteum of the radius parallel to the defect was scraped off. In each rabbit, the defect in one forelimb was treated with the bioactive hydrogel matrix, and the other was treated with a collagen sponge soaked with the bioactive hydrogel matrix. The muscle surrounding the bone defect was sutured closed and the limb tightly wrapped.
[0141]The foreli...
example 3
Effect of Bioactive Hydrogel Matrix on Tendon Re-Growth and Strength
[0142]Four sheep had a 4 mm length of the central portion of the patellar tendon removed from the point of attachment of the tibia to the patella of one leg. A small block of the patella with the attached patellar tendon was also removed. The contra-lateral leg served as the unoperated control. Two defects were filled with a collagen sponge (DuraGen®, Integra LifeSciences) infiltrated with the bioactive hydrogel matrix of the invention, and two defects were filled with a DuraGen® collagen sponge infiltrated with saline. The implants were sutured into the patellar tendon defect and surgical site was sutured closed. After 12 weeks, the patellar tendons were removed for gross observation and mechanical testing for stiffness. The tendons treated with the bioactive hydrogel matrix appeared thicker than the control tendons. Further, the tendons treated with the bioactive hydrogel matrix had an average increase in stiffnes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com