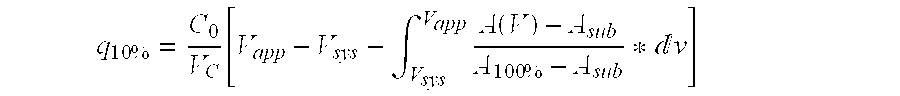Purification of immunoglobulins
a technology of immunoglobulin and purification method, which is applied in the field of antibody preparation, can solve the problems of time-consuming and laborious, impure products, and difficult to use the supernatant for other purposes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n Matrices
[0049] Agarose particles were prepared by suspension gelation as disclosed in U.S. Pat. No. 6,602,990 (Amersham Biosciences). More specifically, particles having a Kav of 0.69 were produced by the appropriate adjustment of solids content, according to well known principles in this field. Further, by adjusting the speed and duration of stirring, the median particle diameter was controlled to 80 μm.
[0050] The particles described above were epoxi-activated and recombinant Protein A (rprotein A) was coupled to the particles via C-terminal, following well known procedures as described e.g. in Hermanson, Greg T., Mallia, A. Krishna, Smith, Paul K. Immobilized Affinity Ligand Techniques, p. 118. Academic Press. ISBN 0-12-342330-9. The rProtein A ligands were coupled to a ligand density of 7.3 mg / ml.
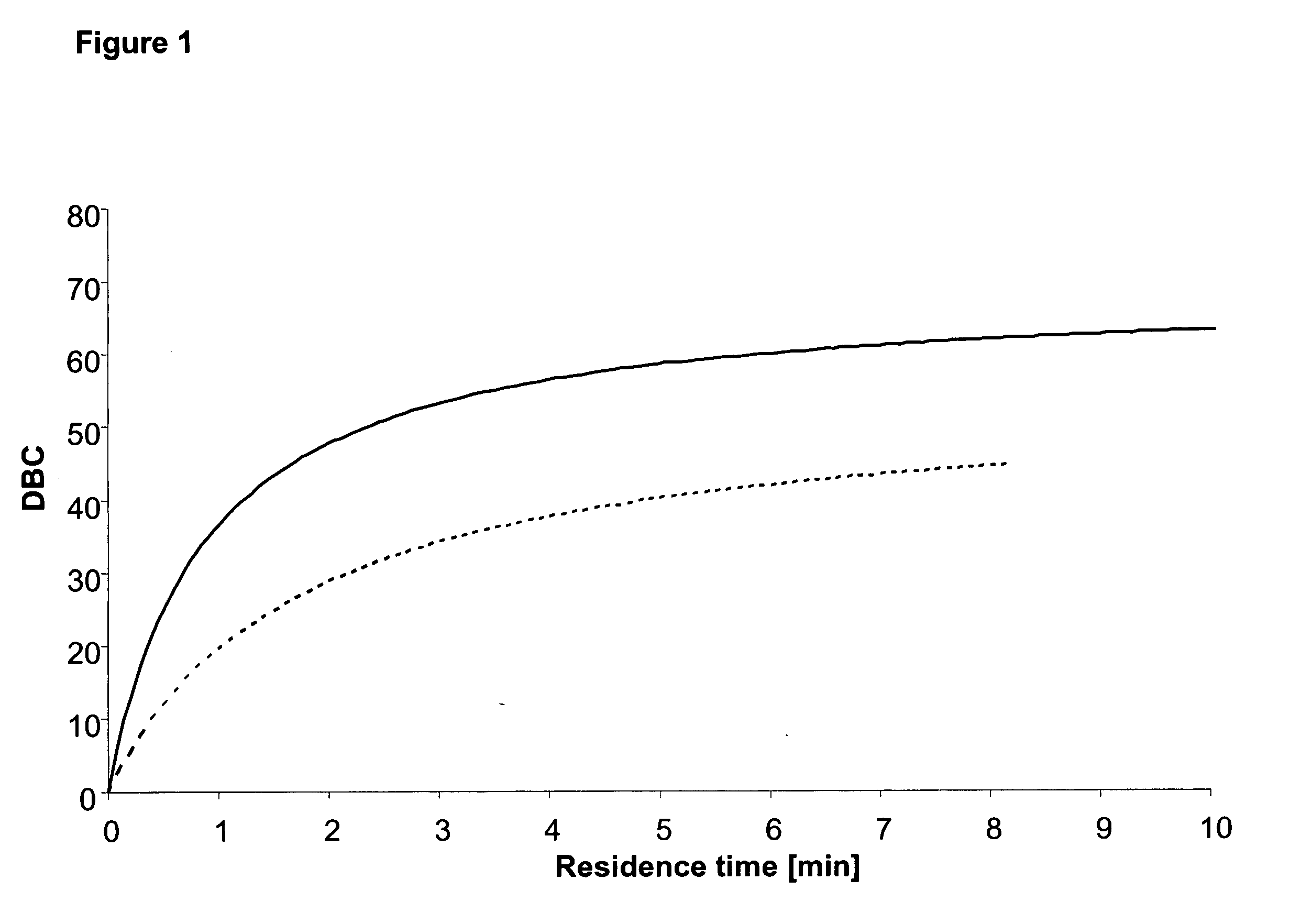
[0051] The comparative separation matrix was MabSelect™, obtained from Amersham Biosciences, which according to the product note presents a median particle size of the cumulative vol...
example 2
inding Capacity
[0052] The dynamic binding capacity (DBC) of the separation matrix prepared as described in Example 1 was tested as follows: Human polyclonal IgG was loaded, 1.0 mg / ml at neutral pH onto two columns packed with the product. The capacity is determined at 10% breakthrough, and the results are shown in FIG. 1.
[0053] Equipment
Packing of columnsColumns (2)XK16 / 20Amersham BiosciencesPacking reservoirXK16 / 20Amersham BiosciencesPump25 ml / minute, e.g. P-900Amersham BiosciencesRelief valve0.3 MPaAmersham BiosciencesPressure meter
[0054]ÄKTA™ Explorer 10 or AKTA FPLC™ (Amersham Biosciences) Spectrophotometer, double beam
ChemicalsEthanol99.5%Spectroph.Sodium Chloridep aSodium Dihydrogenp aBakerM = 137.99 g / molPhosph.Sodium Hydroxidep aProlaboM = 40.00 g / molSodium Citratep aMerckM = 75.07 g / molHydrochloric acidp aGammanorm165 mg / mlOctapharma
Solutions
[0055] Buffers
Packing solution 1:20% (v / v) ethanol containing 0.25 M NaCl.Packing solution 2:20% (v / v) ethano...
example 3
on of the Gel Phase Distribution Coefficient
Principle
[0067] The gel phase distribution coefficient of a particle according to example 1 is determined by gel filtration. Two dextrans with different sizes are run through a packed HR16 / 30 column. Retention volumes for each dextran are detected, and used to calculate Kav, a value describing the fraction of the particle volume available for a certain molecular weight. From the Kav-values the Kav-value for Mp 110000 is reported.
[0068] Equipment
PackingColumnHR 16 / 30Packing tubeHR 16 / 30PumpÄKTA ™ P-900 pump or equal
[0069] Selectivity test
ÄKTA explorer 10 System or equalControlUNICORN ™Sample InjectionAutosampler A-900*Sample loop200 μlPumpsP-900DetectorShimadzu RI-detector
Chemicals
[0070] Mobile phase for packing is distilled water and for the selectivity test 0.20 M NaCl in distilled water.
[0071] The column packing is tested with an injection of 2% acetone in a distilled water mobile phase.
[0072] The dextrans used in the selectiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| median particle diameter | aaaaa | aaaaa |
| median particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com