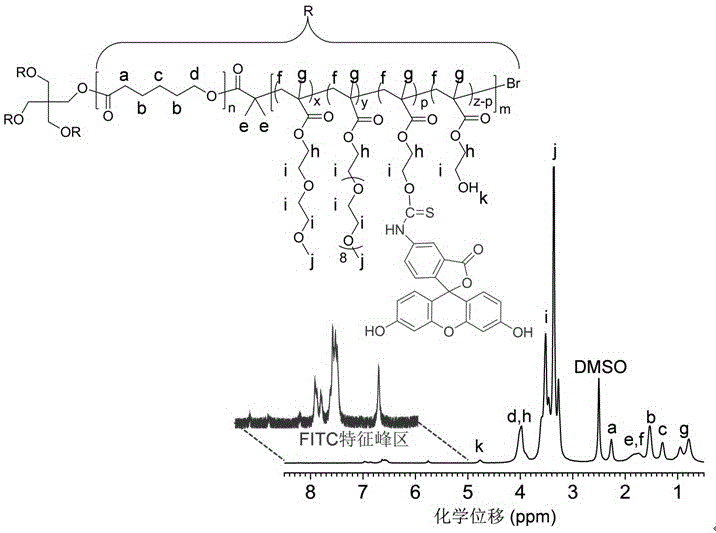
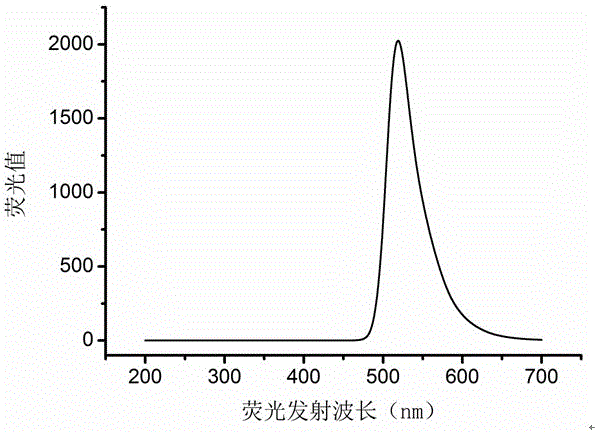
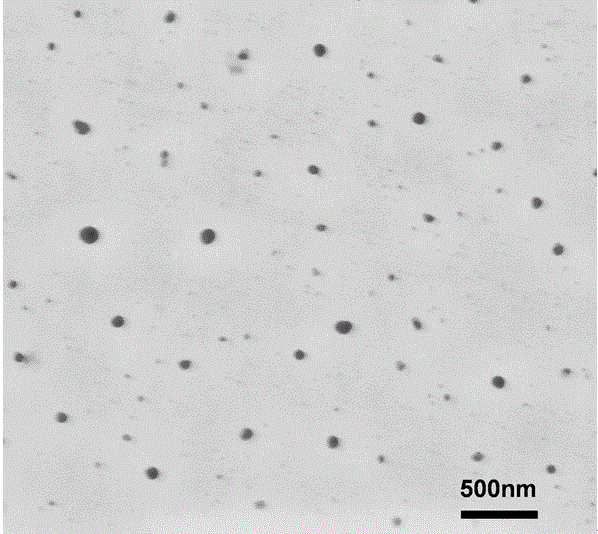
Preparation method of star polymer-based drug carrier material with fluorescence labeling and temperature responsiveness
A temperature-responsive, star-shaped polymer technology, which is applied in the fields of polymer materials and biomedical engineering, can solve problems such as the influence of carrier material drug loading rate, achieve good biocompatibility and temperature responsiveness, and good fluorescent tracer The effect of performance and synthesis method is simple and feasible
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 0.47g of pentaerythritol and 39.4g of ε-caprolactone were added to the reaction flask, and 400μmol of stannous octoate was injected into it. The system was frozen and evacuated three times, reacted at 120°C for 24h under the protection of argon, and dissolved in dichloromethane after cooling. Precipitate with methanol, vacuum and dry to obtain product I star polycaprolactone (4sPCL); Dissolve 17.3g product I and 2.4g triethylamine in 100ml dichloromethane, wait for the system to cool to 0℃, add to it Add 2-bromoisobutyryl bromide solution (4.1g / 25ml CH 2 Cl 2 ), continue to stir for 2h and then move to room temperature to react for 48h. After the reaction is complete, it is concentrated by rotary evaporation and saturated NaHCO 3 The solution was washed with deionized water several times, filtered with anhydrous magnesium sulfate to remove water, precipitated with methanol, filtered and dried in a vacuum to obtain the product II star-shaped macroinitiator (4sPCL-Br); 3....
Embodiment 2
[0033] 0.47g of pentaerythritol and 49.9g of L-lactide were added to the reaction flask, and 300μmol of stannous octoate was injected into it. The system was frozen and evacuated three times, reacted at 110°C for 24h under the protection of nitrogen, and then dissolved in dichloromethane after cooling. Precipitating with methanol, vacuum drying to obtain product I star-shaped polylactic acid (4sPLLA); 21.8 g of product I and 3.1 g of triethylamine were dissolved in 120 ml of dichloromethane, and the system was cooled to 0° C. Add 2-bromoisobutyryl bromide solution (5.5g / 30mlCH 2 Cl 2 ), continue to stir for 2h and then move to room temperature to react for 48h. After the reaction is complete, it is concentrated by rotary evaporation and saturated NaHCO 3 The solution and deionized water are washed several times, dewatered with anhydrous magnesium sulfate, filtered, precipitated with methanol, filtered and dried in vacuum to obtain the product II star-shaped macroinitiator (4sP...
Embodiment 3
[0036] Add 0.38g of dipentaerythritol and 25.7g of ε-caprolactone into the reaction flask, and inject 350μmol of stannous octoate into it, freeze the system and vacuum three times, react at 120°C for 36h under argon protection, and dissolve with dichloromethane after cooling Precipitating with methanol, vacuum drying to obtain product I star polycaprolactone (6sPCL); Dissolve 17.4g product I and 3.1g triethylamine in 80ml dichloromethane, wait the system to cool to 0℃, Among them, 2-bromoisobutyryl bromide solution (6.9g / 40mlCH 2 Cl 2 ), continue to stir for 2h and then move to room temperature to react for 72h. After the reaction is complete, it is concentrated by rotary evaporation and saturated NaHCO 3 The solution was washed with deionized water several times, filtered with anhydrous magnesium sulfate to remove water, precipitated with methanol, filtered and dried in vacuum to obtain the product II star-shaped macroinitiator (6sPCL-Br); 2.9g product II, 10.3g MEO 2 MA, 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com