Synthesis method of neostigmine bromide
A technology of neostigmine bromide and a synthetic method, applied in the field of pharmaceutical synthesis, can solve the problems of not easy to buy, high cost, high production difficulty, etc., and achieves the effects of satisfying industrial mass production, reducing costs, and having sufficient sources of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
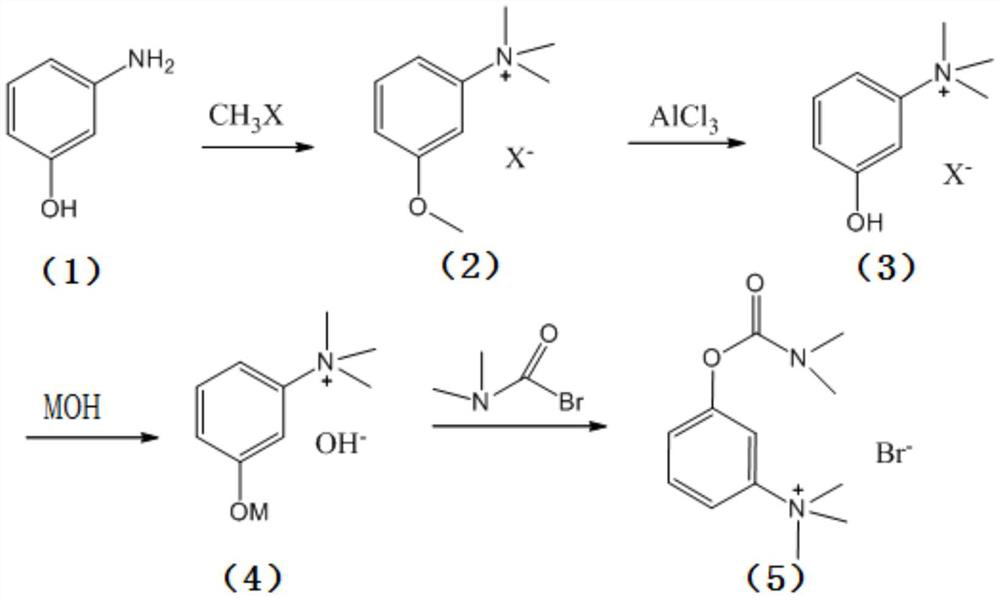
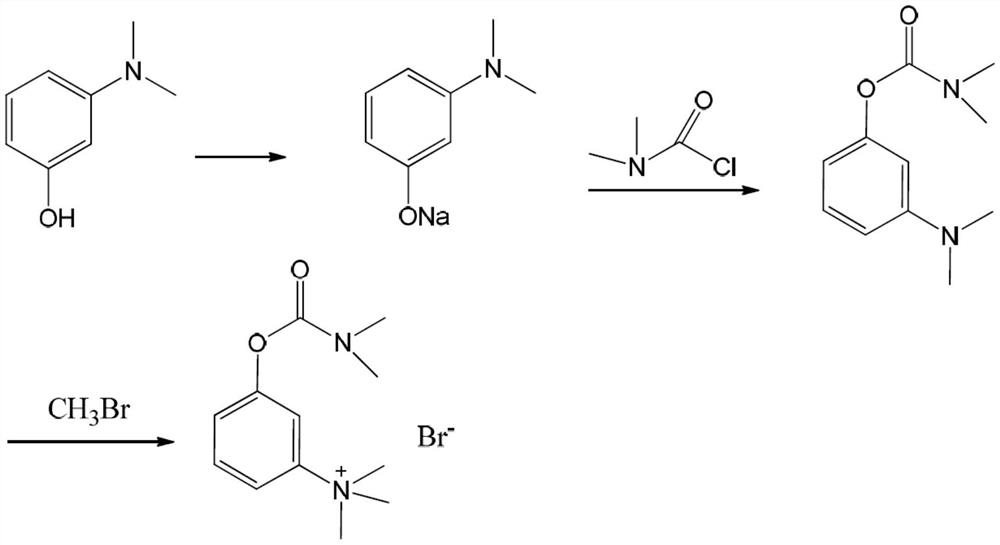
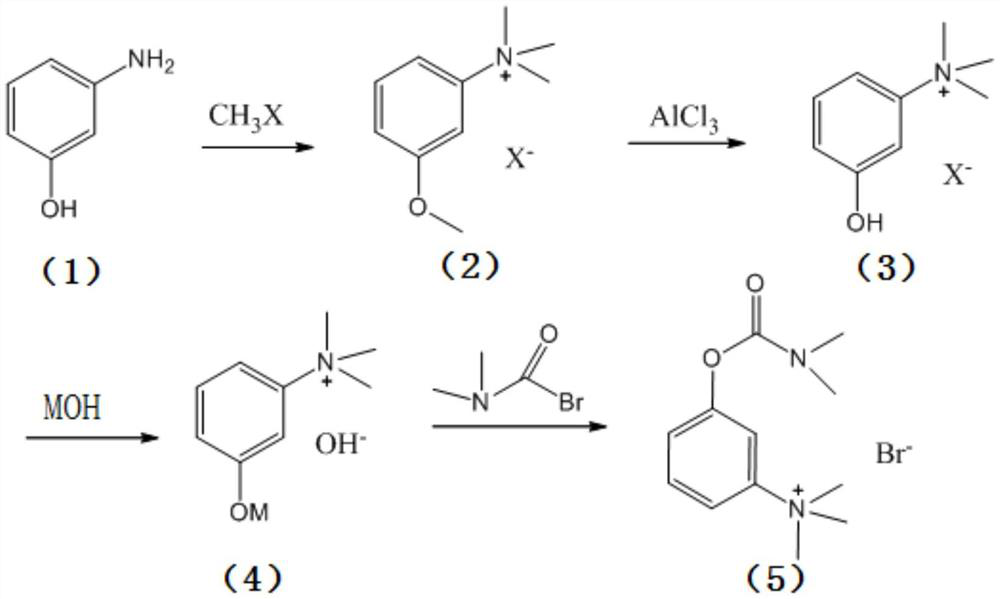
[0028] The present invention provides a kind of synthetic method of neostigmine bromide, the reaction route of described synthetic method comprises:
[0029]
[0030] Wherein, R is an alkali metal;
[0031] The synthetic method comprises the following steps:
[0032] 1) the m-aminophenol of formula (1) is selected to react with methyl halide to obtain the halogenated quaternary ammonium methyl ether of formula (2);
[0033] 2) the halogenated quaternary ammonium salt methyl ether of formula (2) adds catalyzer, and demethylation obtains the m-phenolic hydroxyl quaternary ammonium salt of formula (3);
[0034] 3) the m-phenolic hydroxyl quaternary ammonium salt of formula (3) forms the compound of formula (4) under strong alkali conditions;
[0035] 4) Esterification of the compound of formula (4) with dimethylcarbamoyl bromide to obtain neostigmine bromide of formula (5).
Embodiment 1
[0037] A synthetic method of neostigmine bromide, comprising the following steps:
[0038] 1) In a 10L three-necked flask, add 5L of acetone, 500g of m-aminophenol, and 790g of sodium carbonate, and stir at 25°C for 2 hours. Then, a mixed solution of 1.9 kg of methyl bromide and 1 L of acetone was added dropwise at 25° C., and the mixture was stirred at 25° C. for 4 hours. Concentrate under reduced pressure to recover acetone, add 5L of water for recrystallization. Filter and wash the filter cake with 300ml of ice water. The filter cake was dried under reduced pressure at 60-65° C. to obtain 915 g of quaternary ammonium bromide methyl ether. Purity: 98.3% (HPLC normalization method), yield: 80.0%.
[0039] 2) In a 10L three-necked flask, add 6L of dichloromethane and 800g of bromide quaternary ammonium methyl ether, stir to dissolve, cool to 5°C, slowly add 350g of anhydrous aluminum trichloride in batches, and control the feeding temperature to 25°C. After the addition, t...
Embodiment 2
[0044] A synthetic method of neostigmine bromide, comprising the following steps:
[0045] 1) In a 10L three-necked flask, add 5L of acetone, 500g of m-aminophenol, and then add 560g of sodium hydroxide, and stir at 30°C for 2 hours. Then, at 30°C, a mixed solution consisting of 2.0kg of fluoromethane and 1L of acetone was added dropwise, and the mixture was stirred at 30°C for 5 hours. Concentrate under reduced pressure to recover acetone, add 5L of water for recrystallization. Filter and wash the filter cake with 300ml of ice water. The filter cake was dried under reduced pressure at 65° C. to obtain 930 g of fluoroquaternary ammonium methyl ether. Purity 98.8% (HPLC normalization method), yield: 81.7%;
[0046]2) In a 10L three-necked flask, add 6L of dichloromethane and 800g of fluoroquaternary ammonium methyl ether, stir to dissolve, cool to 0°C, slowly add 350g of anhydrous aluminum trichloride in batches, and control the feeding temperature to 20°C. After the additi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com