Use of galantamine for treatment of neuropsychiatric behaviour associated with alzheimer's disease
A technology for Alzheimer's and neuropsychiatric diseases, applied to neuropsychiatric behavioral drugs, to treat neuropsychiatric behaviors related to Alzheimer's disease, and can solve problems such as galantamine that has not been confirmed by research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
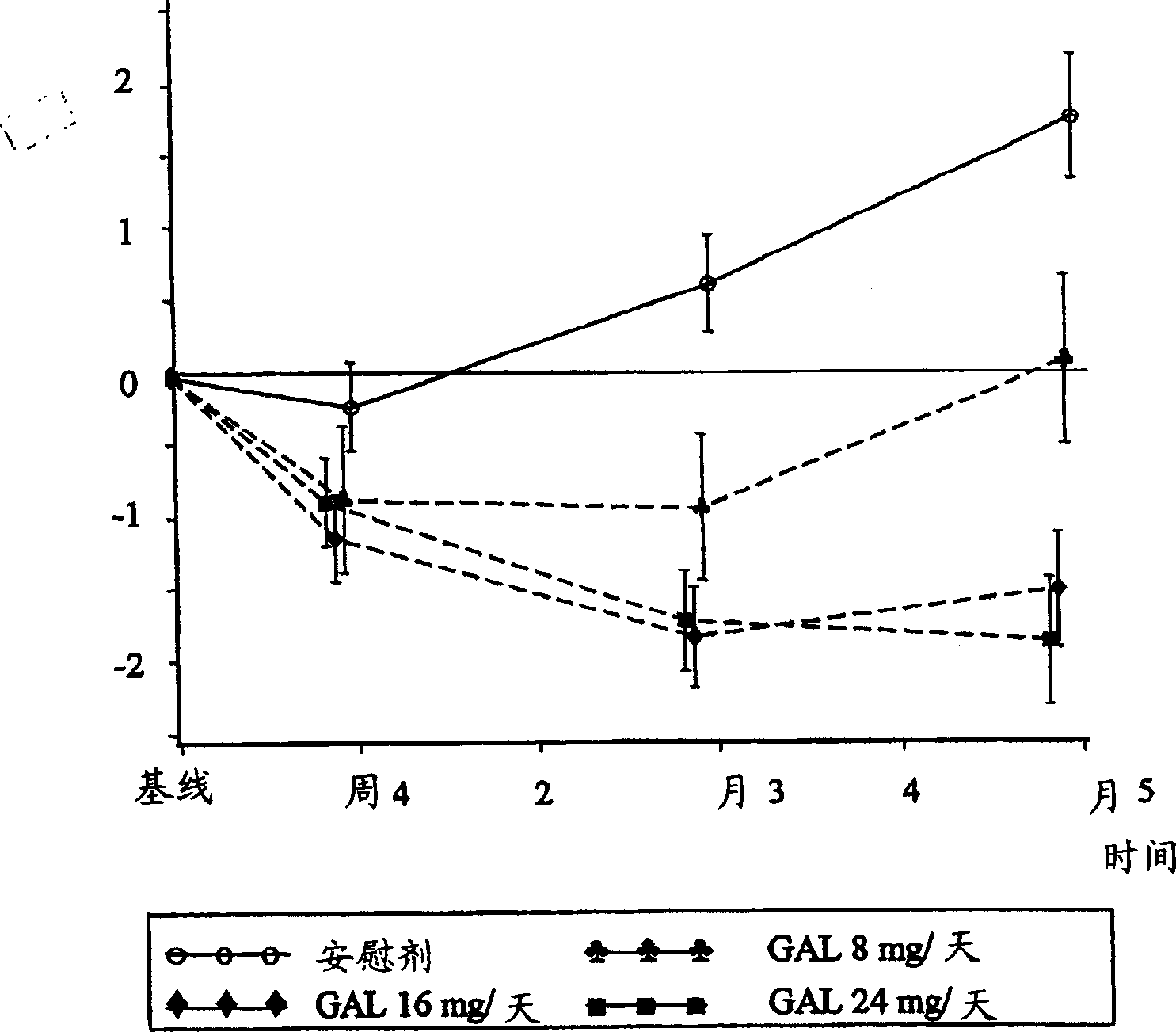
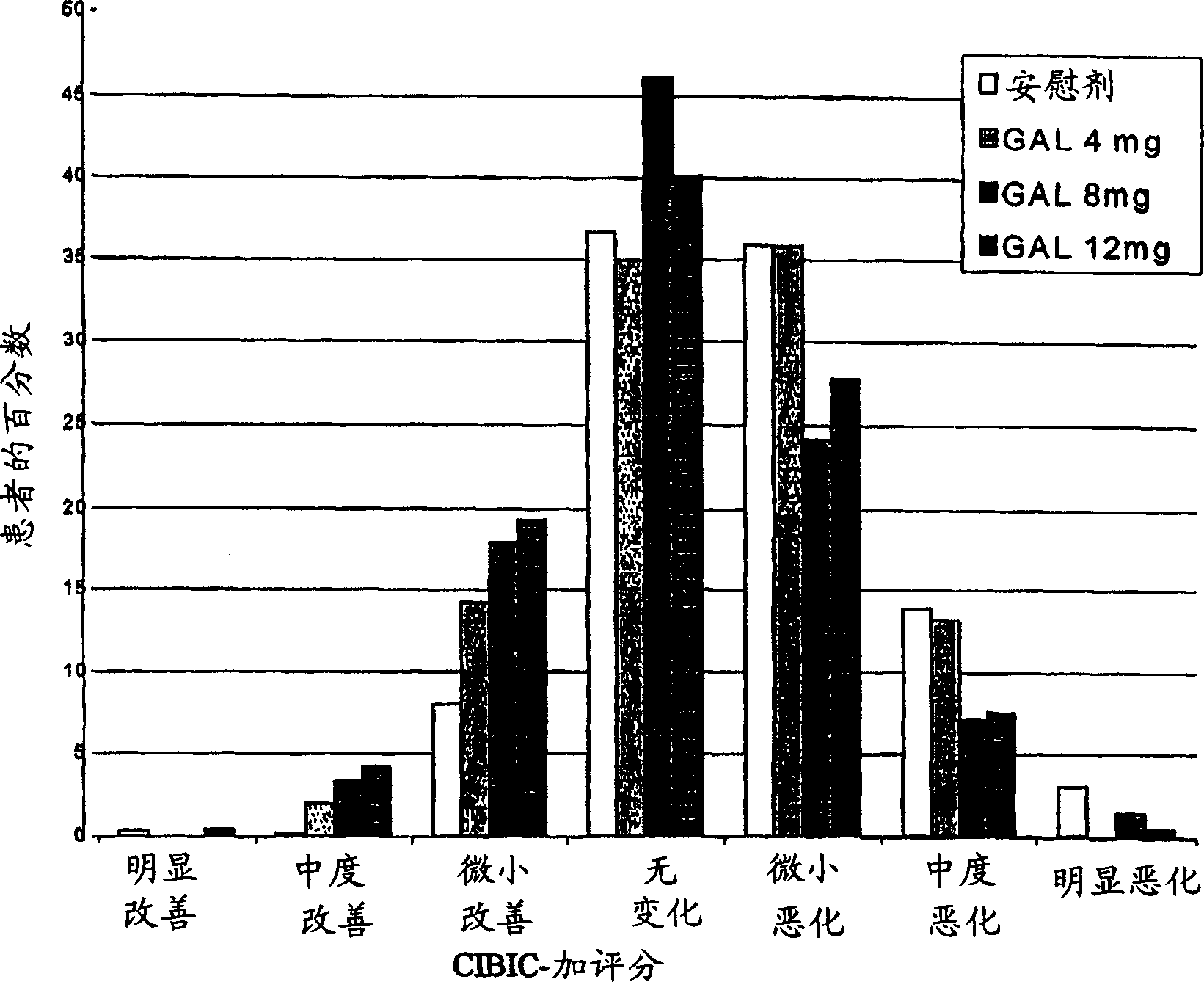
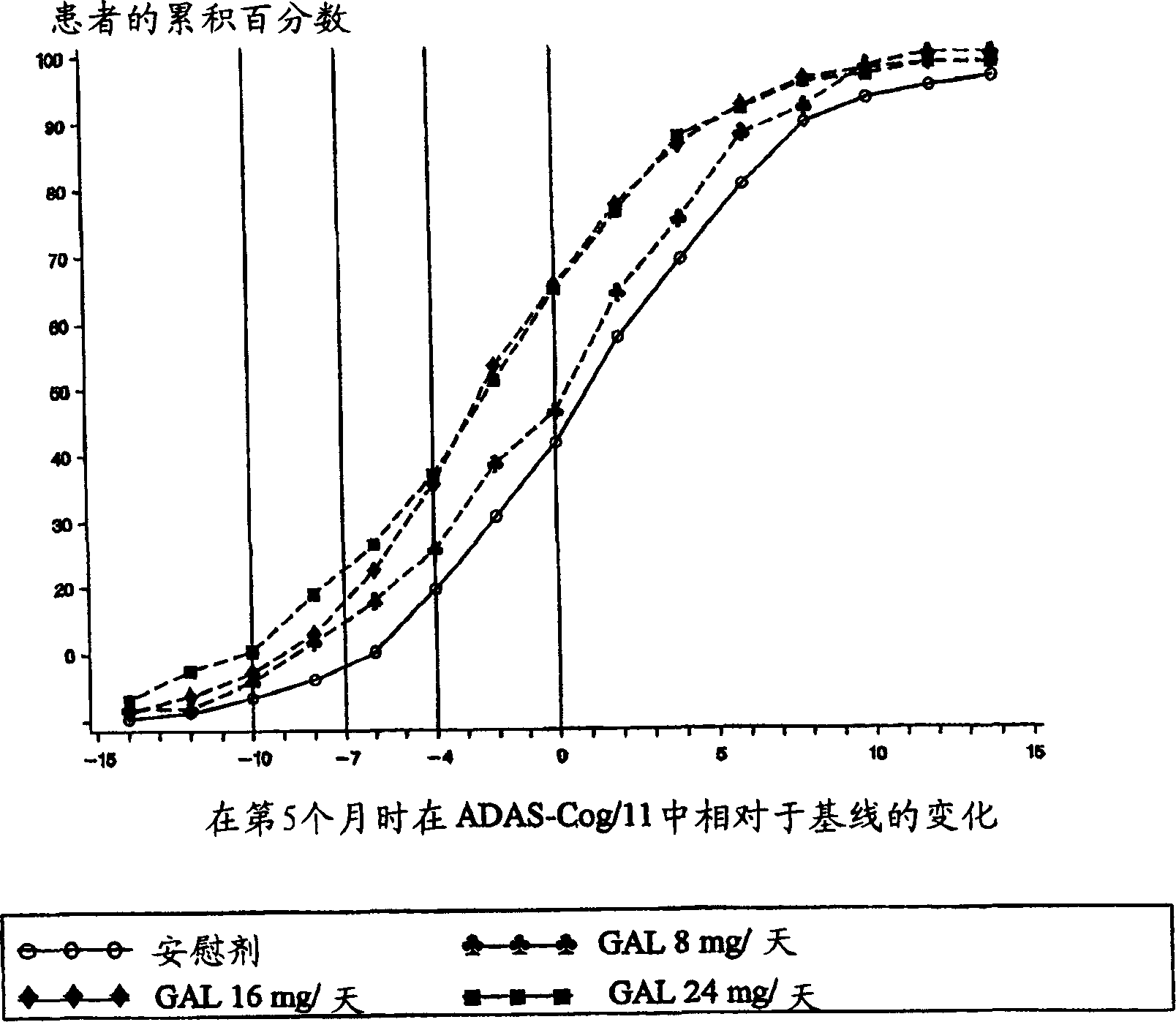
[0033] Patients (approximately 910) diagnosed with Alzheimer's disease were randomly divided into 4 treatment groups: placebo; gradually increased dose to galantamine 24 mg / day over 8 weeks; gradually increased dose to Galantamine 16mg / day; or galantamine 8mg / day, the dose is no longer gradually increased, and a total of 5 months of treatment. Patients enrolled in the study must have been diagnosed with Alzheimer's disease, Alzheimer's disease assessment grade (Rosen, W.G. et al., Amer.J.Psychiatry, 141:1356-1364, 1984) cognition Score on section (ADAS-cog-11) of at least 18 and a history of cognitive decline that was progressive at the beginning and developed over a period of at least 6 months.
[0034] The dose escalation scheme for each treatment group was as follows:
[0035] Subjects in the placebo group received placebo treatment for 21 weeks (5 months). The subjects in the Gal24 group received 4 weeks of 8mg / day galantamine (4mg, twice a day (bid)), 4 weeks of 16mg / da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com