Galantamine clearance of amyloid-beta
A technology of amyloid and galantamine, which is applied in the directions of medical preparations containing active ingredients, drug combinations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
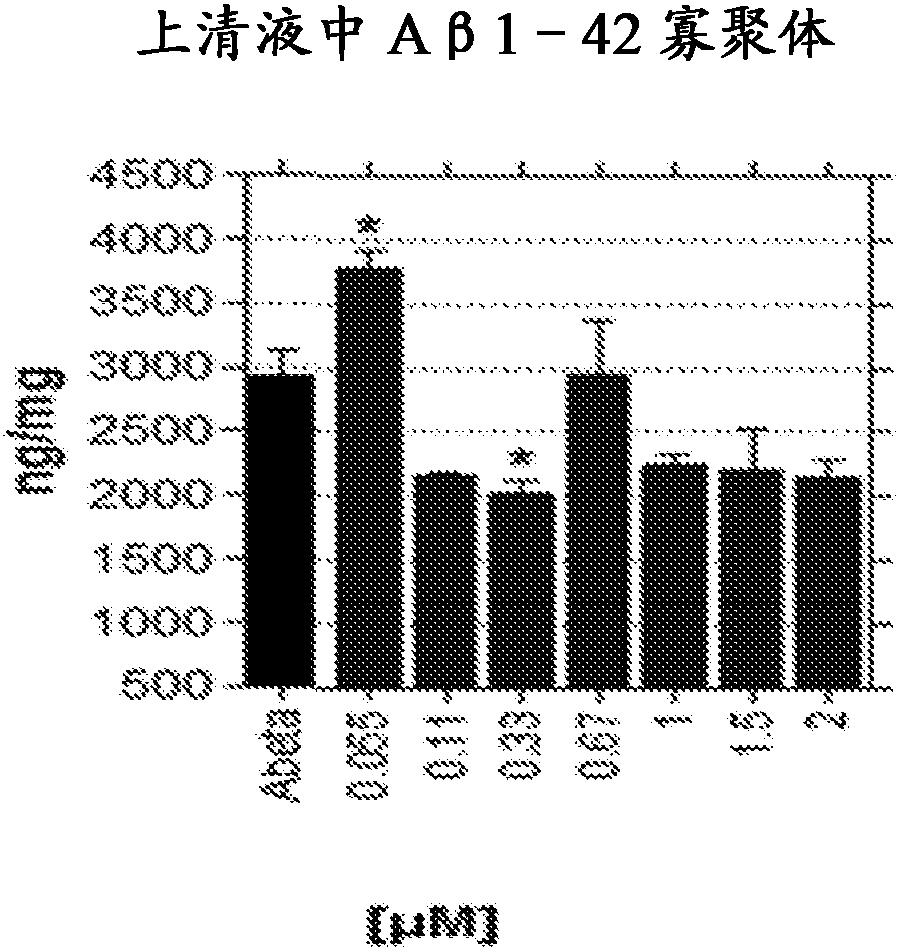
[0081] A method for determining an appropriate dose range for galantamine can be achieved by evaluating in vitro the concentration that improves Aβ oligomer clearance, and doing so with a concentration that protects the neurite network from residual oligomer damage. adjust. Galantamine or a pharmaceutically acceptable salt is then administered to experimental animals to determine plasma and brain concentrations, and to apply plasma concentrations related to effective brain concentrations to human subjects. To determine appropriate doses of galantamine for groups of subjects with varying degrees of cognitive impairment, counter-regulatory increases in CSF acetylcholinesterase can be determined. The dose of galantamine will be determined when the increase in CSF acetylcholinesterase does not exceed the increase in CSF acetylcholinesterase when 16 to 24 mg of galantamine is administered to a subject with Alzheimer's dementia Suitable, as demonstrated by Nordberg et al., 2009, su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com