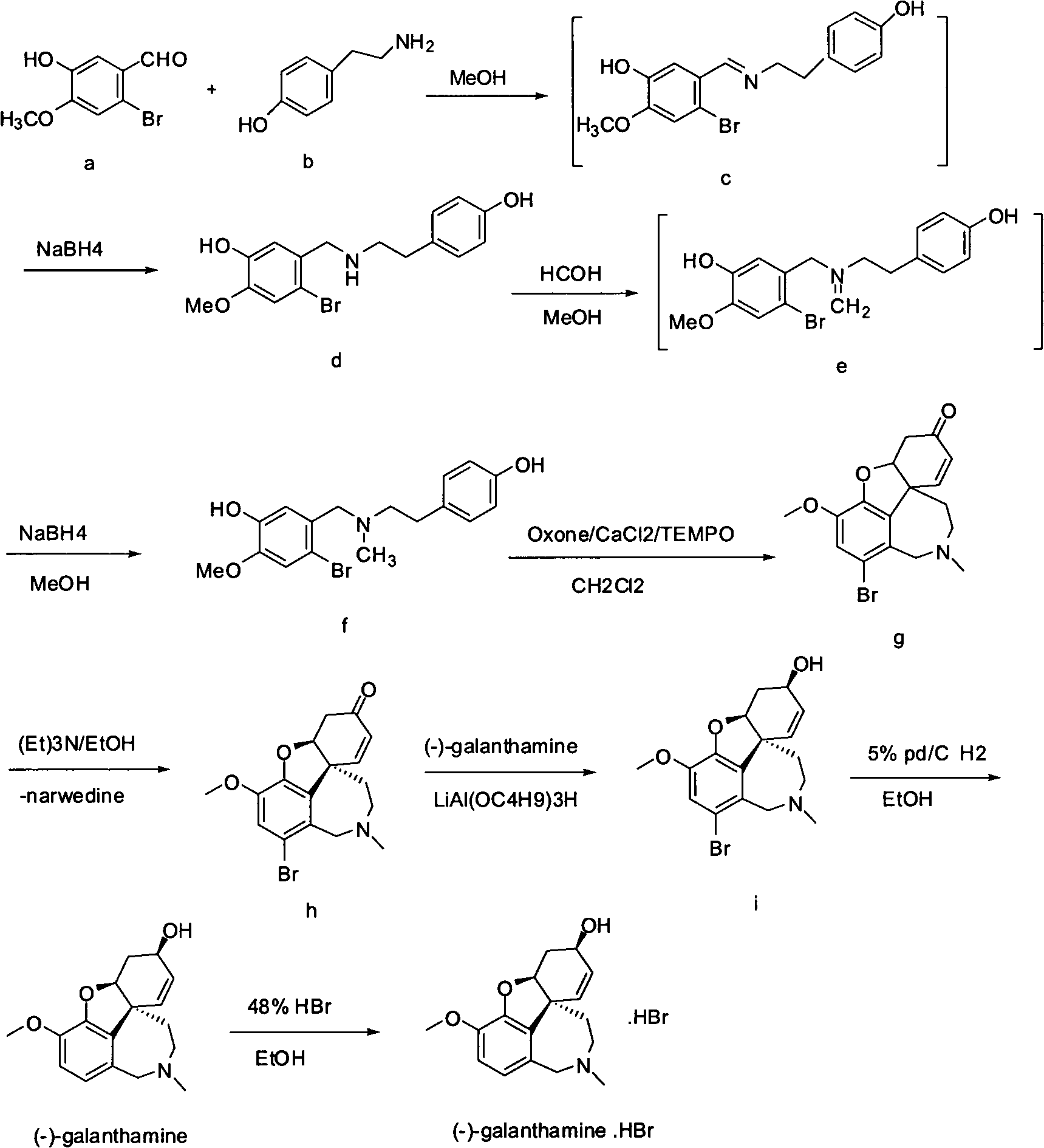
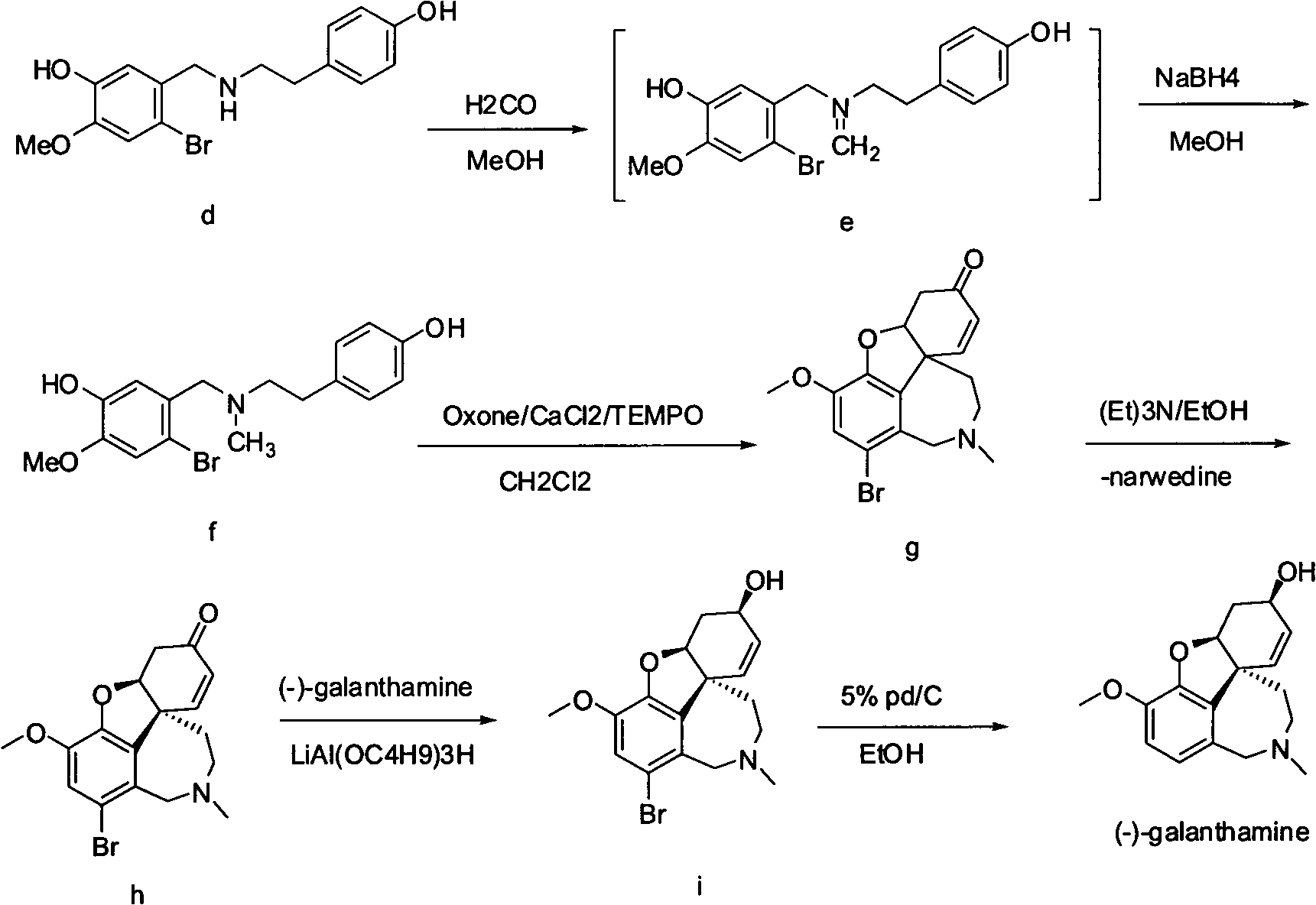
Synthesis method of galanthamine
A technology of galantamine and hydroxyl, applied in the field of synthesis of galantamine, can solve the problems of high price of galantamine, only 1.4%, high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] N-(4-hydroxyphenethyl)-3-hydroxy-4-methoxy-6-bromobenzylamine (d)
[0025] 58g (0.25mol) of 6-bromoisovanillin (a) was dissolved in 280ml of methanol, 35g (0.25mol) of tyramine (b) was added at room temperature, and the reaction was stirred for 1 hour. Cool down to below 0°C, add 8.5g (0.225mol) sodium borohydride in batches, stir at 0°C for 1 hour, and stir at room temperature for 3 hours, evaporate the solvent under reduced pressure, add dichloromethane to dissolve, wash with water, and evaporate to dryness to obtain (d )75g, yield 85.2%.
Embodiment 2
[0027] N-methyl-N-(4-hydroxyphenethyl)-3-hydroxy-4-methoxy-6-bromobenzylamine (f)
[0028] Dissolve 50g (0.14mol) (d) in 250ml methanol, add 32ml of 37% formaldehyde, react at room temperature for 2 hours, cool down to 0°C, add 4.8g (0.126mol) sodium borohydride, react at 0°C for 3 hours, add 50ml The solid was precipitated in ice water and filtered to obtain 41.4 g of (f), with a yield of 80.6%.
Embodiment 3
[0030] Bronavirdine (g)
[0031] Dissolve 20g (0.055mol) (f) in 200ml of dichloromethane, add 1g of tetrabutylammonium bisulfate and appropriate amount of water at room temperature, add oxidant OXone (33g, 0.055mol), CaCl 2 (610.5mg, 5.5mmol), TEMPO (77mg, 0.55mmol), stirred at room temperature for 2 hours, stirred at 40°C for 2 hours, washed with water, concentrated to one-third volume, precipitated solid, stirred at room temperature for 3 hours, filtered to obtain ( g) 12.4 g, yield 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com