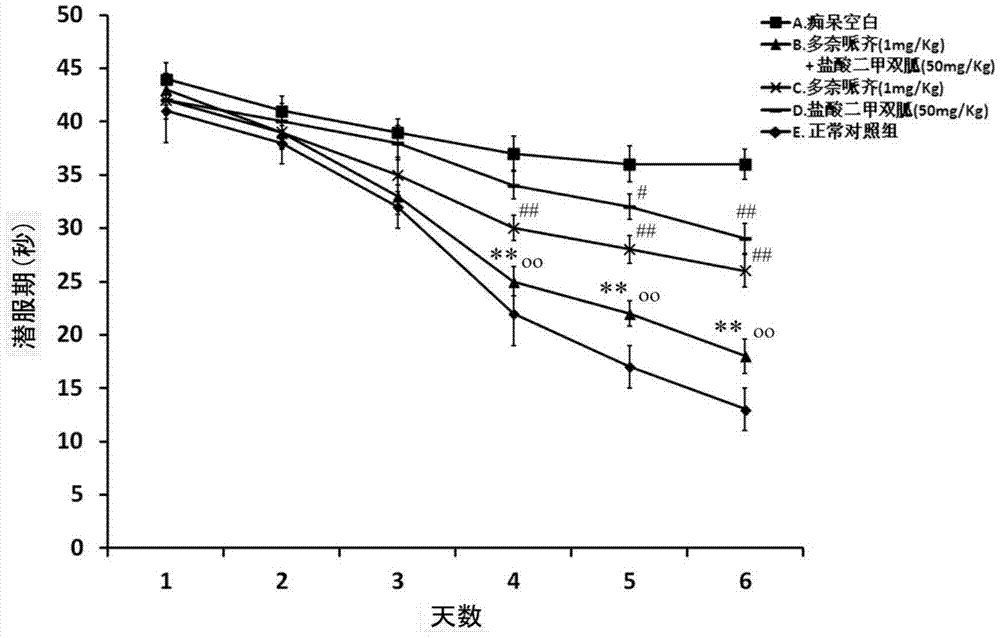
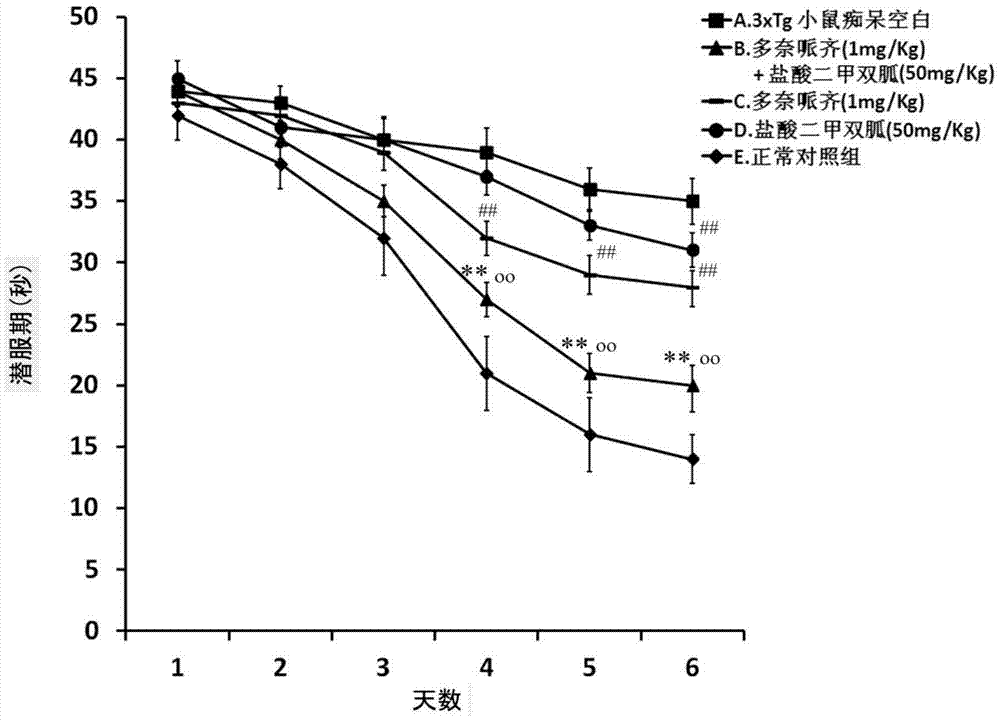
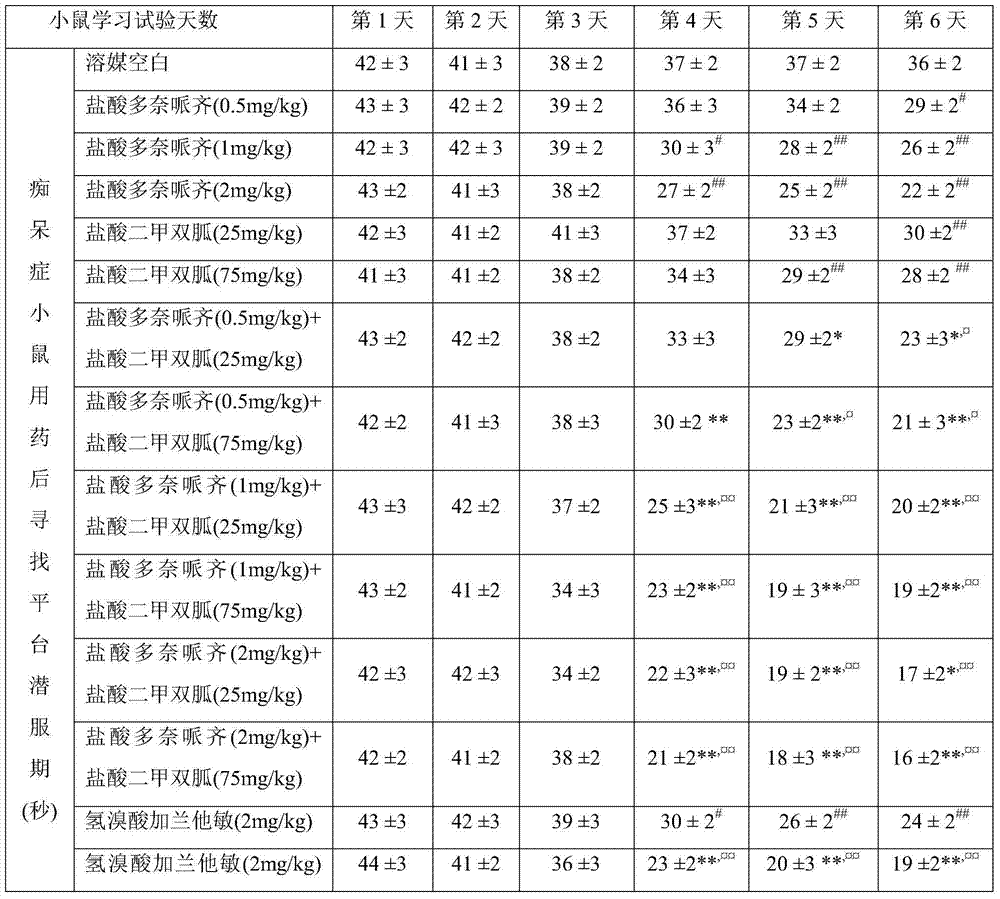
Compound medicinal composition containing acetylcholinesterase inhibitor and metformin
A technology of acetylcholinesterase and metformin, applied in the field of medicine, can solve the problems of inability to improve AD nerve cell function, deterioration of cognitive impairment, brain nerve cell death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Donepezil Hydrochloride and Metformin Hydrochloride Compound Tablets
[0036] Prescription: Ordinary Tablets (Based on 1000 Tablets)
[0037] components
Dosage
5g
Metformin Hydrochloride
500g
45g
10g
4g
2% hypromellose (solid weight)
6g
[0038] Preparation:
[0039] (1) Pass donepezil hydrochloride, metformin hydrochloride, and microcrystalline cellulose through an 80-mesh sieve respectively, and after uniformly mixing by an equal-volume increasing method, premix with 2% hypromellose and wet granulate;
[0040] (2) drying and sizing the granules prepared by wet granulation to obtain dry granules;
[0041] (3) blending dry granules with croscarmellose sodium and magnesium stearate to obtain blended granules;
[0042] (4) Compressing the blended granules into tablets to obtain tablets.
Embodiment 2
[0044] Donepezil Hydrochloride and Metformin Hydrochloride Bilayer Tablets
[0045] Prescription: double layer tablet (according to 1000 tablets)
[0046]
[0047] Preparation:
[0048] (1) Pass donepezil hydrochloride, microcrystalline cellulose, and starch through an 80-mesh sieve respectively, and after mixing uniformly by the method of equal increments, premix with 2% hypromellose and perform wet granulation;
[0049] (2) Metformin hydrochloride and microcrystalline cellulose were respectively passed through an 80-mesh sieve, mixed evenly by an equal-volume increasing method, premixed with 2% hypromellose and wet-granulated;
[0050] (3) drying and sizing the A-layer and B-layer granules prepared by wet granulation, respectively, to obtain the dried granules of the A-layer and B-layer;
[0051] (4) The dry granules of A layer and B layer are blended with croscarmellose sodium and magnesium stearate respectively to obtain the blended granules of A layer and B layer;
...
Embodiment 3
[0054] Donepezil Hydrochloride and Metformin Hydrochloride Capsules
[0055] Prescription: Capsules (according to 1000 capsules)
[0056] components
Dosage
5g
250g
30g
Magnesium stearate
10g
2% hypromellose (solid weight)
10g
[0057] Preparation:
[0058] (1) Pass donepezil hydrochloride, metformin hydrochloride and microcrystalline cellulose through an 80-mesh sieve respectively, mix uniformly by the method of equal volume increment, premix with 2% hypromellose and wet granulate.
[0059] (2) drying and sizing the granules prepared by wet granulation to obtain dry granules;
[0060] (3) dry granules are mixed with magnesium stearate to obtain mixed granules;
[0061] (4) Fill the blended granules into capsules with a capsule filling machine to obtain capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com