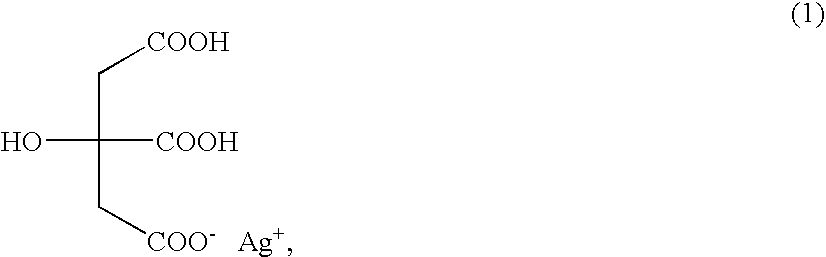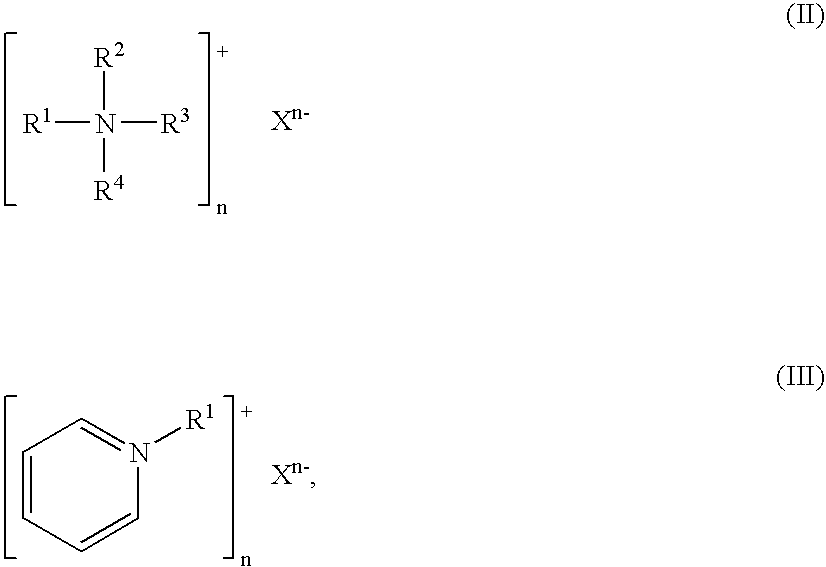Silver dihydrogen citrate compositions comprising a second antimicrobial agent
a technology of silver dihydrogen citrate and composition, applied in the field of antimicrobial compositions, can solve the problems of prolonging the antimicrobial effect, irritating or even toxic, and not being effective against some gram-negative bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of Silver Dihydrogen Citrate Stock Solution
[0053] Water was introduced into a reverse osmosis unit, passing through a semi-permeable membrane to remove impurities and producing deionized water. Anhydrous 99% pure citric acid was mixed with the water to produce 200 gallons of a 20% (wt / vol) (796 g citric acid per gallon water) solution. The 200 gallons of 20% citric acid were directed into an ion chamber containing having positive and negative electrodes, each consisting of 200 troy ounces of 999 fine silver. The positive and negative electrodes were spaced at least 2.0 mm apart, allowing the citric acid solution to pass between the two electrodes. An ion generation controller (IGC) power supply including a positive and a negative conductor was attached to the positive and negative electrodes. The IGC applied a current of 5 amps at 17 volts, pulsed every 9 seconds, with a polarity change at 1 minute intervals. Throughout the process, the electrode gap was adjusted in orde...
example 2
Production of a Composition of Silver Dihydrogen Citrate and a Quaternary Ammonium Compound, an Oxidizing Agent or a Halogen Compound
[0055] (a) A scented, colored intermediate composition: In a 1,000 ml Pyrex™ glass flask, there were combined the ingredients set forth in Table 1 in the indicated proportions:
TABLE 1IngredientProportionPure Pharmaceutical-Grade98.882%WaterQuaternary Ammonia 80%0.300%ConcentrateTriton X-100 ™ surfactant0.500%Non-toxic Scent0.300%Non-toxic color0.015%
[0056] (b) Scented, non-colored composition: In a 1,000 ml Pyrex™ glass flask, there were combined the ingredients listed in Table 2 in the indicated proportions:
TABLE 2IngredientProportionPure Pharmaceutical-Grade98.897%WaterQuaternary Ammonia 80%0.300%ConcentrateTriton X-100 ™ surfactant0.500%Non-toxic Scent0.300%
[0057] (c) Non-scented, colored composition: In a 1,000 ml Pyrex™ glass flask, there were combined the ingredients listed in Table 3 in the indicated proportions:
TABLE 3IngredientProportio...
example 3
Antimicrobial Effect of the Composition from Example 3
[0060] An E. coli test strain (E. coli PIPSA, German H.) was grown at 35° C. for 24 hours. The cells were harvested by centrifugation for 10 minutes and were washed twice with Butterfield's Phosphate Buffer (BPB pH. 7.2). The cells were then re-suspended in BPB to obtain a cell suspension of approximately 108 CFU / ml. (Target inoculum levels were approximately 106 CFU / ml in the final test solution).
[0061] Three sets of two non-porous glass slides were provided. For each set of test slides, one was treated with the test solution in Example 3 and the other slide was treated with the intermediate test solution from Example 3. Each slide was then inoculated with E. coli.
[0062] After inoculation, the test slides were then stored for periods of 1 and 24 hours, then plated out at the indicated time period and incubated for a period of 24 hours. A score of Pass indicates no bacterial growth. A score of Fail indicates bacterial growth. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com