A kind of preparation method of insulin sustained-release oral preparation
A technology for oral preparations and insulin, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of subcutaneous fat atrophy, inconvenience and pain of patients, etc. Controllable appearance, good biocompatibility and water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] 1. Preparation process
[0030] 1. Synthesis of PEGylated Chitosan
[0031] Weigh 1g CS (CS is chitosan, molecular weight 500000), dissolve it in 6 ml acetic acid, the volume concentration of acetic acid (v / v) is 2%, dilute it with 20 ml TEMED•HCL, add it into a three-necked flask, and measure the pH The value is about 5, then add a certain amount of EDC and NHS, after 30 minutes, weigh mPEG-COOH according to the molar ratio of mPEG-COOH:CS=1:1, add it to a three-necked flask, keep the constant temperature water bath at 35°C, and stir for 36 hours. pH 4-6 was controlled during the reaction.
[0032] The obtained crude product was placed in a dialysis bag for dialysis for 4 days, and freeze-dried to obtain PEGylated chitosan.
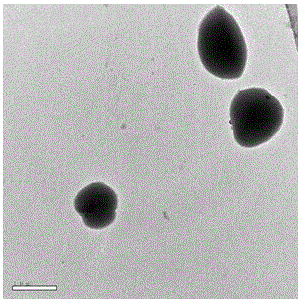
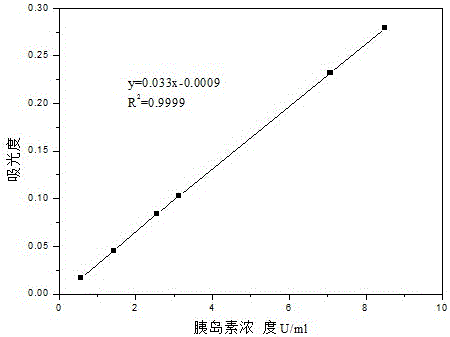
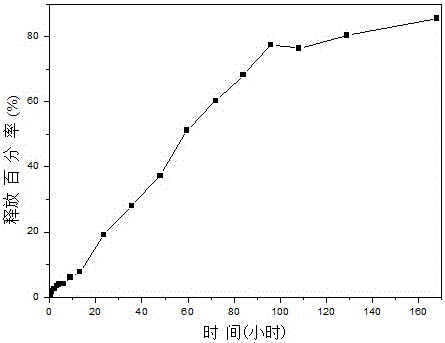
[0033] 2. Preparation of Insulin-Loaded PEGylated Chitosan Microspheres
[0034] (1) Take a certain amount of PEG-CS (polyethylene glycolated chitosan) and TPP (sodium tripolyphosphate) and dissolve them in distilled water respectively to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com