Gastric acid secretion inhibiting composition
a technology of gastric acid and composition, applied in the direction of drug composition, dispersed delivery, antibacterial agents, etc., can solve the problems of not providing symptom resolution, e. complete relief of symptoms, etc., and achieve the effect of quick and lasting reli
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
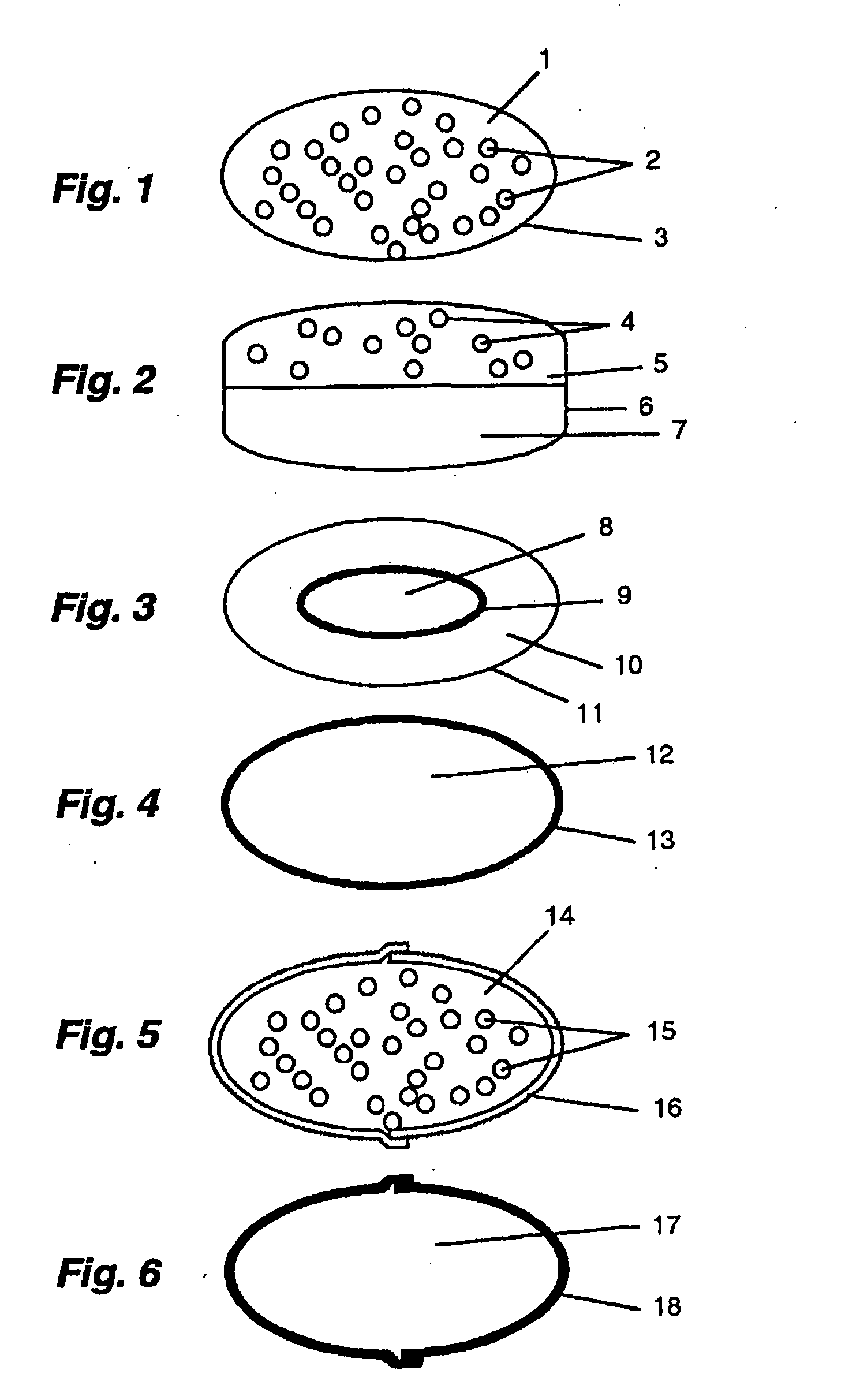
[0069] Multiple unit tableted dosage form comprising magnesium omeprazole and ranitidine hydrochloride; batch size 400 tablets. For omeprazole Mg-salt pellet production (core material, separating layer, enteric coating layer and over-coating layer, see WO 97 / 25066, p. 22-23 under respective headings), see WO 97 / 25066, first two paragraphs, all of which is hereby incorporated by reference.
TabletsPrepared pellets comprising omeprazole Mg-salt31.3gMicrocrystalline cellulose300.0gCimetidine hydrochloride40.0gPotato starch50.0gWater200.0gPVP crosslinked38.0gSodium stearyl fumarate4.6g
[0070] A small amount of the potato starch is dissolved in purified hot water to form the granulation liquid. Cimetidine hydrochloride, the rest of potato starch and microcrystalline cellulose are dry mixed. The granulation liquid is added to the dry mixture and the mass is wet mixed. The wet mass is dried in an oven at 50° C. The prepared granulation is milled through sieve 1 mm in an oscillating mill equ...
example 2
[0072] Three-layered tableted dosage form. The tablet comprises the acid susceptible proton pump inhibitor omeprazole, a separating layer and a core layer comprising cimetidine hydrochloride. Batch size 1000 tablets.
First tablet layerCimetidine hydrochloride200.0gMicrocrystalline cellulose250.0gPVP crosslinked13.0gSodium stearyl fumarate3.8gSeparating layerMicrocrystalline cellulose80.0gSecond tablet layerEnteric coating layered pellets comprising78.3gomeprazole magnesium salt (same as inEXAMPLE 1)Microcrystalline cellulose174.0gPVP crosslinked26.0gSodium stearyl fumarate1.4g
[0073] The constituents of the first tablet layer are dry mixed and precompressed as a first layer in a tableting machine equipped with oval punches. Microcrystalline cellulose is filled on the top of the first layer to form a separating layer to the next layer. The constituents of the second tablet layer are dry mixed and filled on top of the separating layer. The three layers are compressed into a three laye...
example 3
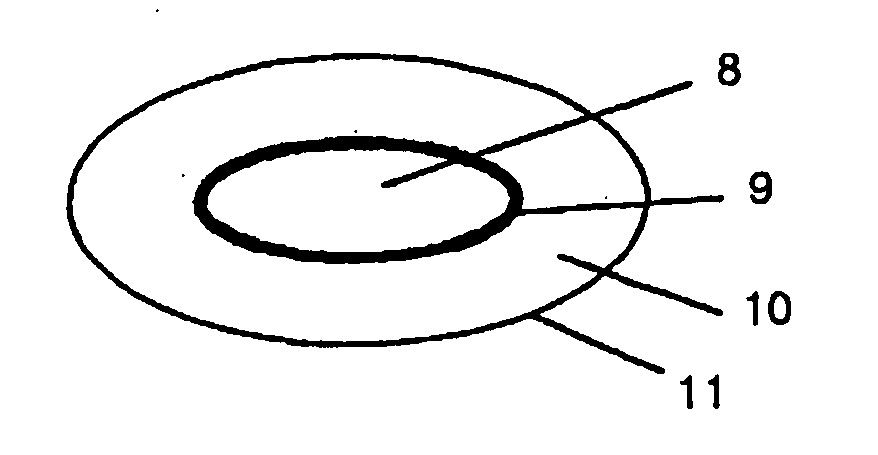
[0074] Capsule dosage form. No. 1 hard gelatin capsules (16) (FIG. 5; volume 0.48 ml) were filled with enteric coated omeprazole pellets (15) containing 20 mg omeprazole recovered from commercially available omeprazole (Losec®) capsules and a dry mixture 14 of commercially available famotidine 20 mg for injection (Pepcidin®; containing 20 mg famotidine hydrochloride, 8 mg aspartic acid and 40 mg mannitol), and closed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
| time period | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com