Oral suspension of liposome-encapsulated insulin lyophilized preparation and preparation process thereof
A freeze-dried preparation, insulin technology, applied in the field of medicine, can solve the problems of low bioavailability, unsolvable storage and transportation problems, poor stability of dosage form production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
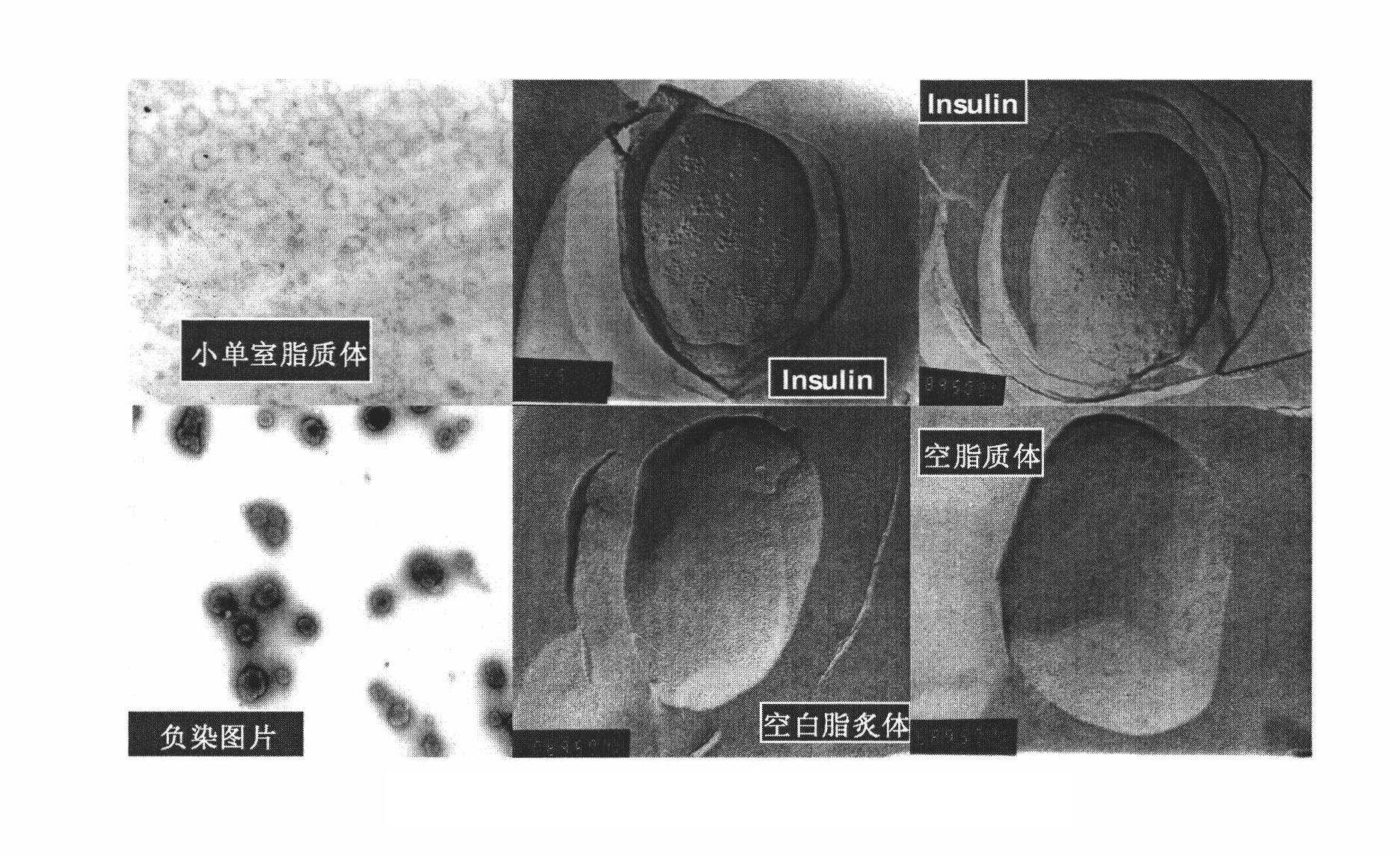
[0111] (1) Preparation of small unilamellar liposome suspension: 1563.2 mg of soybean lecithin, 204 mg of cholesterol, 122.76 mg of distearoyl polyethylene glycol, dissolved in 80 ml of refined chloroform containing 210 mg of vitamin E, placed in In a 250ml round-bottomed flask, evaporate to dryness at 45°C and 200rpm for 3.5 hours. After fully drying at room temperature, add 80ml of distilled water to fully hydrate overnight. The instrument (Particle Sizer) measures the particle size of the small single-chamber liposome to stop after 40-120 nanometers, and leave it at room temperature;
[0112] (2) Preparation of insulin solution: dissolve 700 mg of natural biological insulin dry powder (porcine insulin) in 80 ml of acidic (pH=2.5) aqueous solution, and then adjust the pH to 7.2 with NaOH solution;
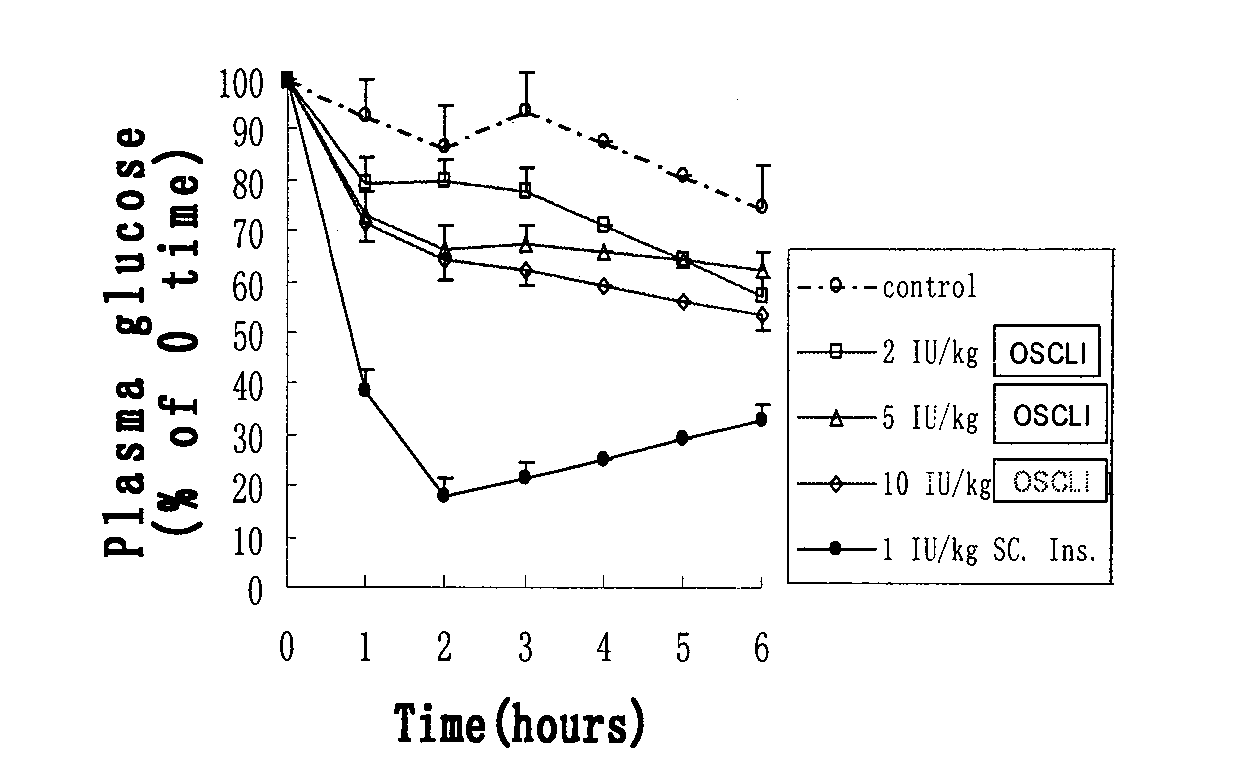
[0113] (3) Preparation of small unilamellar phospholipid liposome-encapsulated insulin freeze-dried preparation: small unilamellar liposome solution (step 1) and insulin solution...
Embodiment example 2
[0116] (1) Preparation of small unilamellar liposomes: 647.56 mg of soybean lecithin, 153 mg of cholesterol, 368.28 mg of polyethylene glycol distearic acid, dissolved in 40 ml of refined chloroform containing 73 mg of vitamin E, and placed in a 250 ml round In the bottom flask, 45°C, 200rpm rotary evaporation for 3.5 hours evaporated to dryness, fully dried at room temperature, added 40ml of distilled water to fully hydrate overnight, after repeated vibrations, ultrasonicated with a probe ultrasonic instrument for 5-22 minutes, and then used a laser scattering instrument (Particle Sizer) measure the small unicellular liposome particle diameter to 40-120 nanometers and then stop, and leave it at room temperature;
[0117] (2) Preparation of insulin solution: dissolve 400 mg of dry insulin powder (porcine insulin) in 40 ml of acidic aqueous solution, and then adjust the pH to 7.2 with NaOH solution;
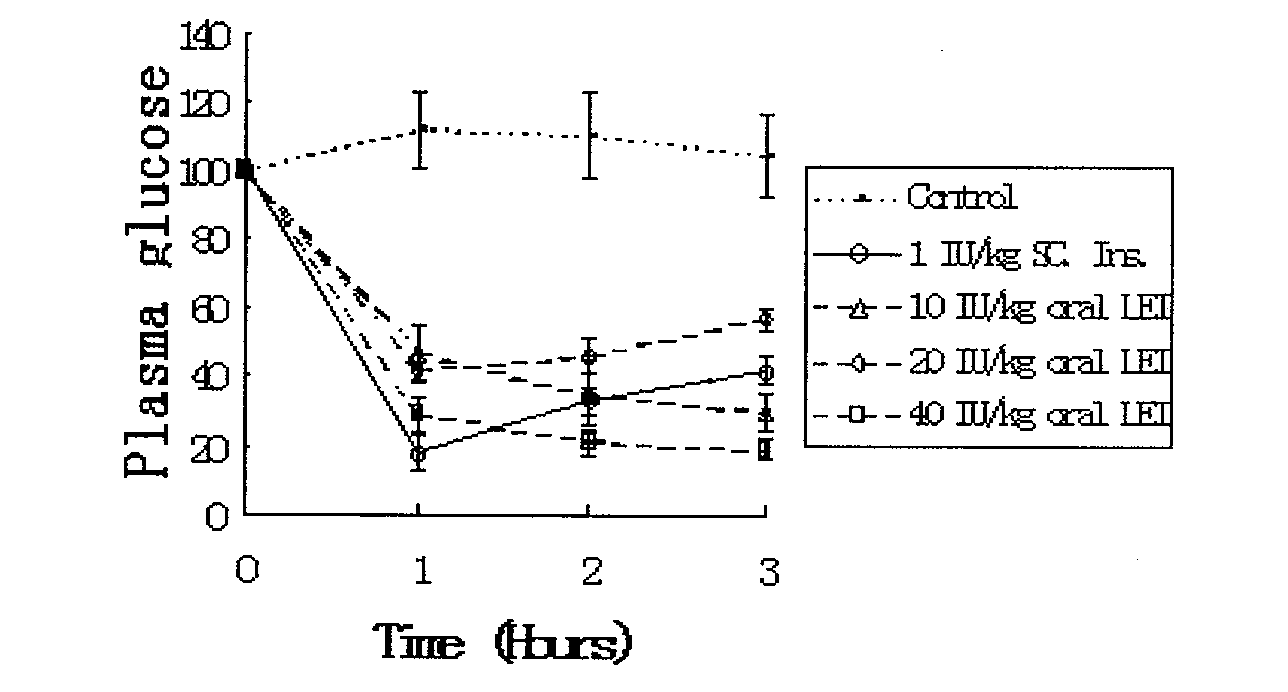
[0118] (3) Preparation of small unilamellar liposome-encapsulated insulin fre...
Embodiment example 3
[0121] (1) Preparation of small unilamellar liposomes: 781.6 mg of soybean lecithin, 102 mg of cholesterol, 245.6 mg of distearoyl polyethylene glycol, added to 40 ml of refined chloroform containing 80 mg of vitamin E, dissolved in 250 ml of In the round bottom flask, 45°C, 200rpm rotary evaporation for 2 hours evaporate to dryness, after fully drying at room temperature, add 40ml of distilled water to fully hydrate overnight. ParticleSizer) measures the small unicellular liposome particle diameter and stops after 40-120 nanometers, and leaves standstill at room temperature;
[0122] (2) Preparation of insulin solution: 400 mg of natural biological insulin dry powder (porcine insulin) was dissolved in 40 m] of acidic aqueous solution, and then adjusted to pH 7.2 with NaOH solution;
[0123] (3) Prepare a lyophilized formulation of insulin encapsulated in small unilamellar phospholipid liposomes. The above-mentioned small unilamellar liposome solution (1) is fully mixed with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com