Azithromycin dosage forms with reduced side effects
A technology of azithromycin and dosage form, applied in the field of azithromycin dosage form with reduced side effects, can solve problems such as low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
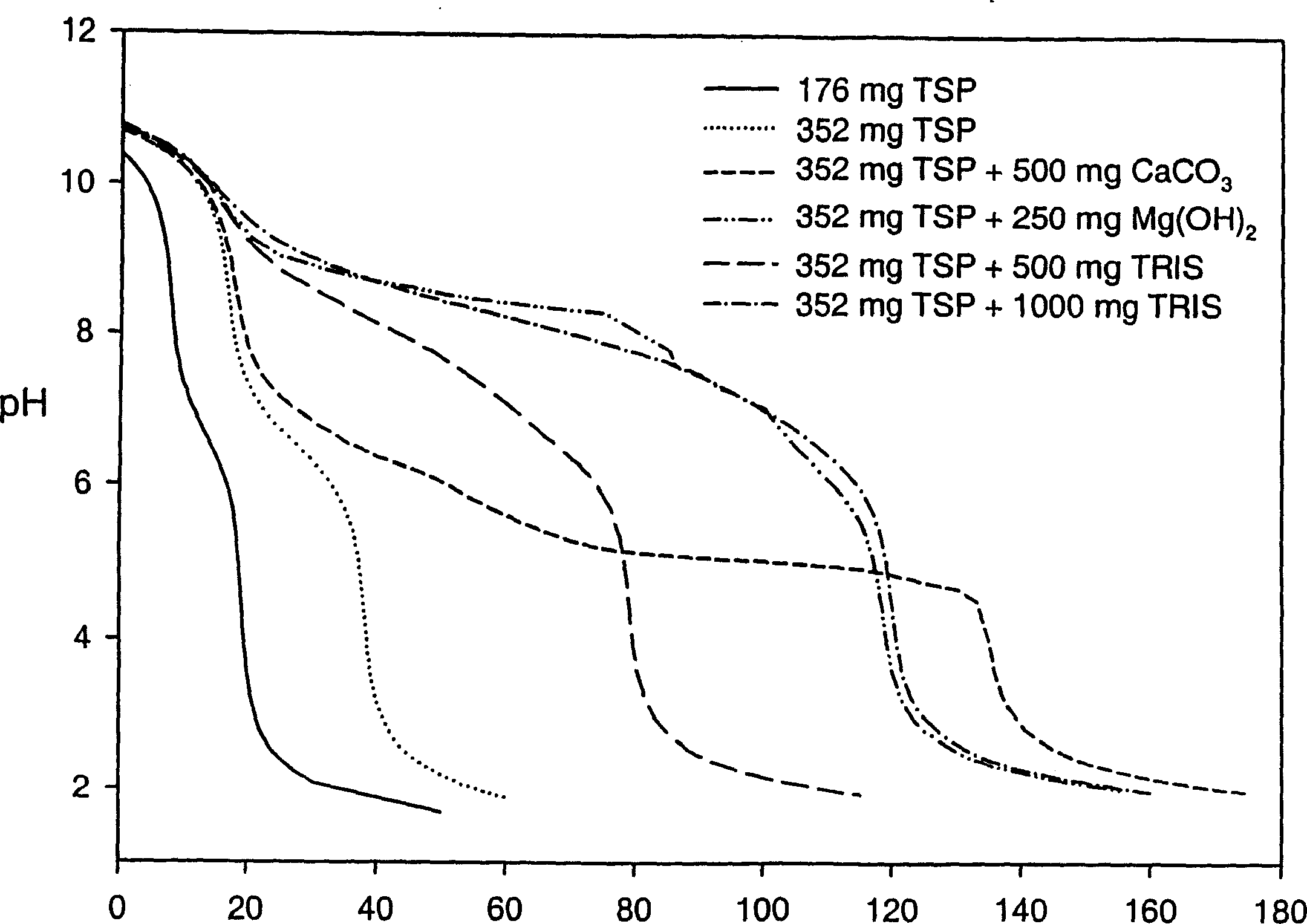
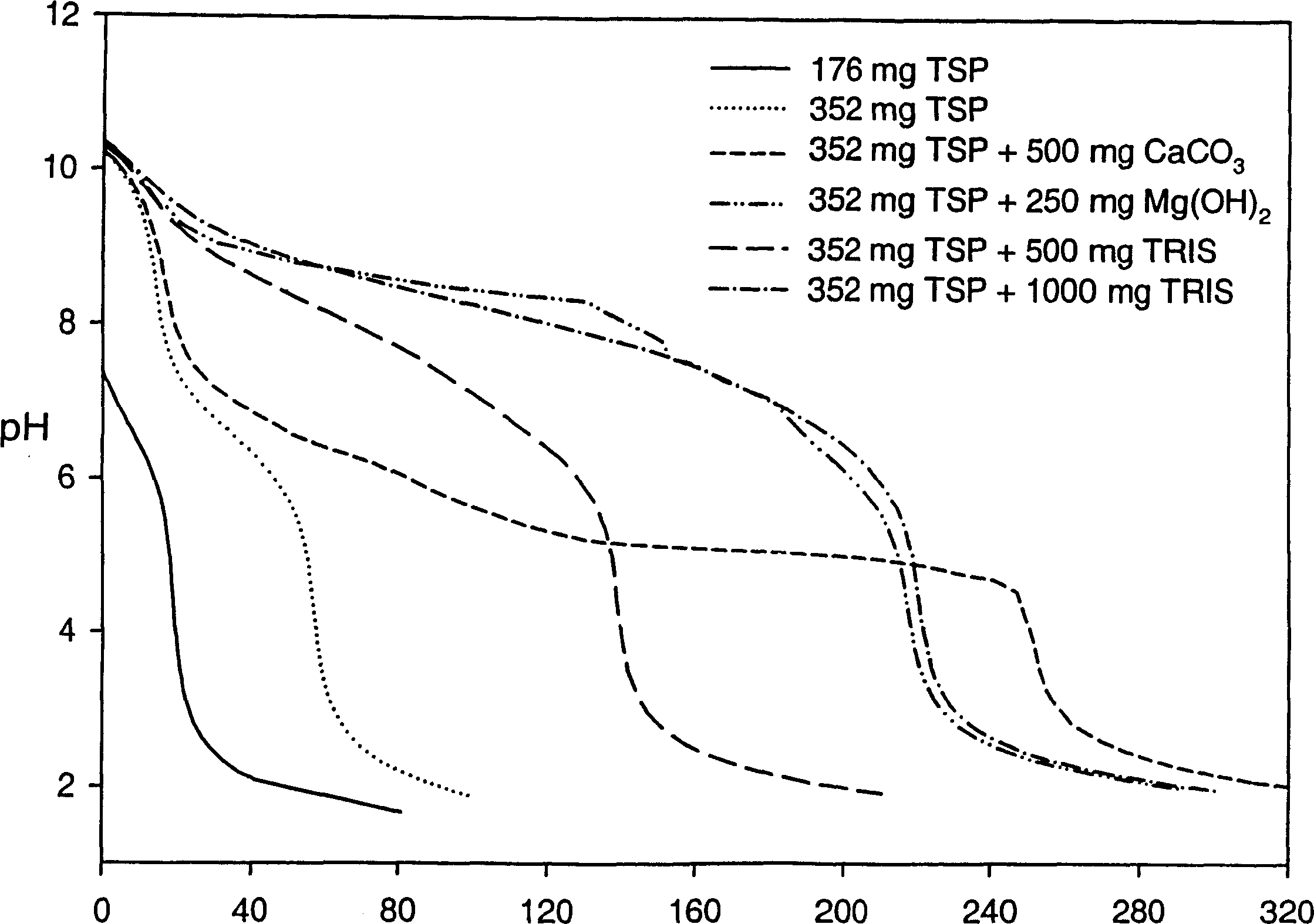
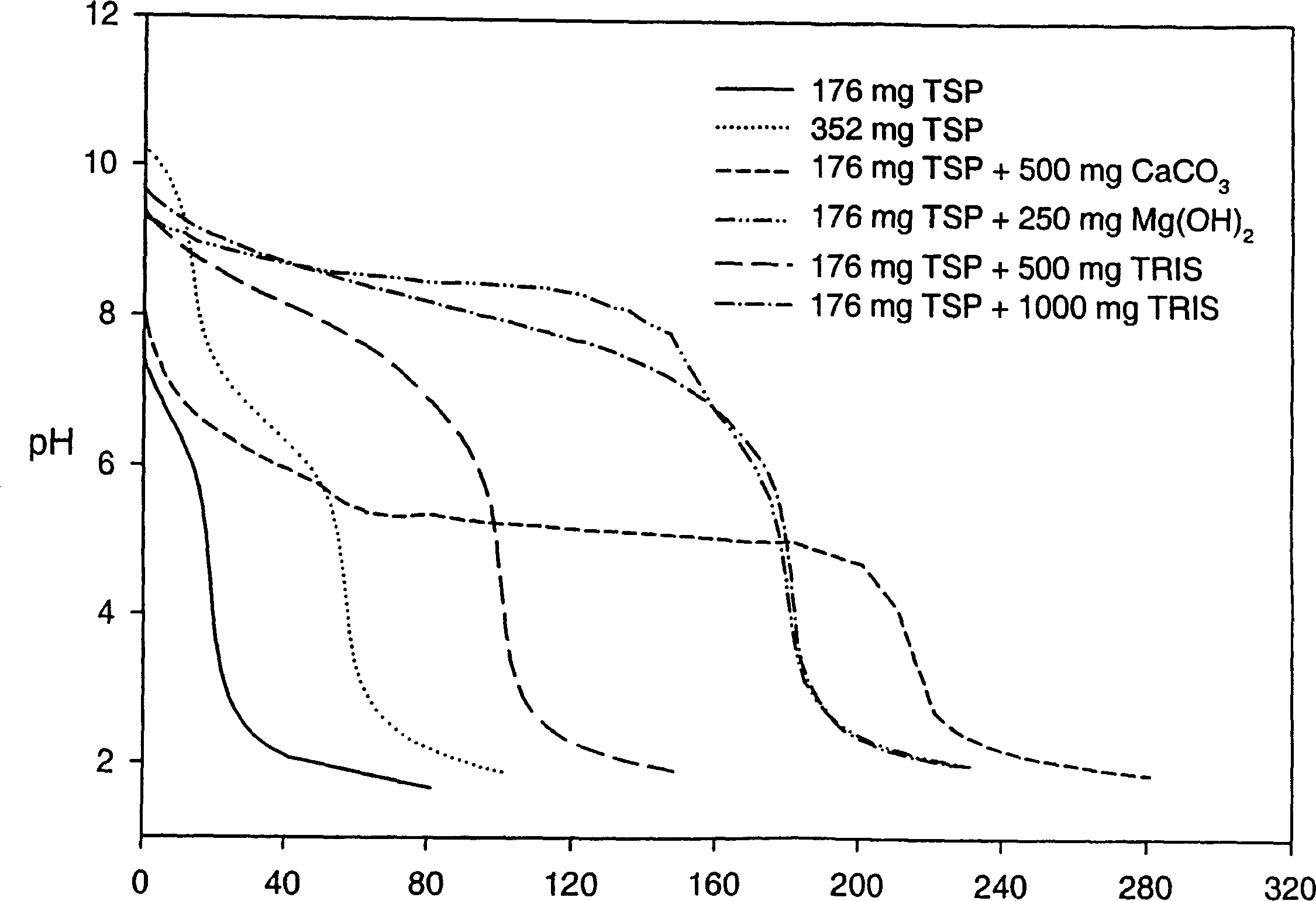
[0214] Effects of Various Alkalizing Reagents on Gastric pH
[0215] A clinical study was performed to monitor gastric pH (using a pH probe) after administration of six different formulations containing an alkalizing agent. Prior to conducting this clinical study, a titration study was performed on the formulation containing the basifying agent to determine the change in pH upon addition of 0.1 N HCl to the basifying agent.
[0216] The formulations tested included the following alkalizing agents:
[0217] Formulation 1-176 mg TSP anhydrous
[0218] Formulation 2-352 mg TSP anhydrous
[0219] Formulation 3 - 352 mg anhydrous TSP and 500 mg calcium carbonate
[0220] Formulation 4 - 352 mg anhydrous TSP and 250 mg magnesium hydroxide
[0221] Formulation 5-352 mg anhydrous TSP and 500 mg tromethamine (TRIS)
[0222] Formulation 6 - 352 mg anhydrous TSP and 1000 mg tromethamine (TRIS)
[0223] In addition, by mixing a specific alkalizing agent with 19.36g sucrose, 0.067g...
Embodiment 2
[0245] Dosage forms of the same but different amounts of alkalizing reagent
[0246] Comparison of release rates in vitro
[0247] Various azithromycin ER dosage forms containing 2 gA of the same azithromycin multiparticulate (MP1) and different amounts of TSP as alkalizing agent were tested compared to azithromycin dosage forms containing MP1 multiparticulates without TSP and azithromycin immediate release dosage forms containing TSP in vitro release rate. Prepare sustained-release dosage forms as described in Step A below, and perform in vitro release rate studies and measure the results as described in Step B below.
[0248] Step A - Preparation of Azithromycin Sustained Release Dosage Form
[0249] Five sustained-release dosage forms of azithromycin (hereinafter denoted "SR1", "SR2", "SR3", "SR4", "SR5") were prepared by mixing 2000 mgA of azithromycin multiparticulate MP1 with one of six excipient mixtures, The preparation method and five dosage forms are described...
Embodiment 3
[0271] Comparison of In Vitro Release Rates of Dosage Forms with Different Basifying Agents
[0272] The in vitro release rate of azithromycin was determined in 0.01N HCl from various azithromycin extended-release dosage forms, each containing 2gA azithromycin multiparticulate MP1 prepared from one of three excipient mixtures, as described below:
[0273] "SR6" includes 38.7g sucrose and 100mg sodium carbonate weak base,
[0274] "SR7" includes 38.7g sucrose and 50mg magnesium hydroxide, and
[0275] "SR8" consists of 38.7g sucrose and 1.0g Liquid Maalox(R) (smooth cherry (smooth cherry), regular strength, from Novartis), which contains 37.1mg aluminum hydroxide, 37.1mg magnesium hydroxide and 3.7mg simethicone ).
[0276] The release rate of azithromycin from these extended release dosage forms was measured as described in Example 2. The results of these dissolution tests, shown in Table 2 below, show that the addition of various basifying agents slowed the release rate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com