Methods for producing an oralsuspension of teichoplanin or teichoplanin analogs
a technology of teichoplanin and oral suspension, which is applied in the directions of peptide delivery, emulsion delivery, saccharide peptide ingredients, etc., can solve the problems of inconvenient reconstitution process for both the pharmacist and the patient in need of teichoplanin treatment, incomplete administration of dose, and decreased patient compliance, so as to improve patient compliance, taste and stability, and reduce the ability to swallow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
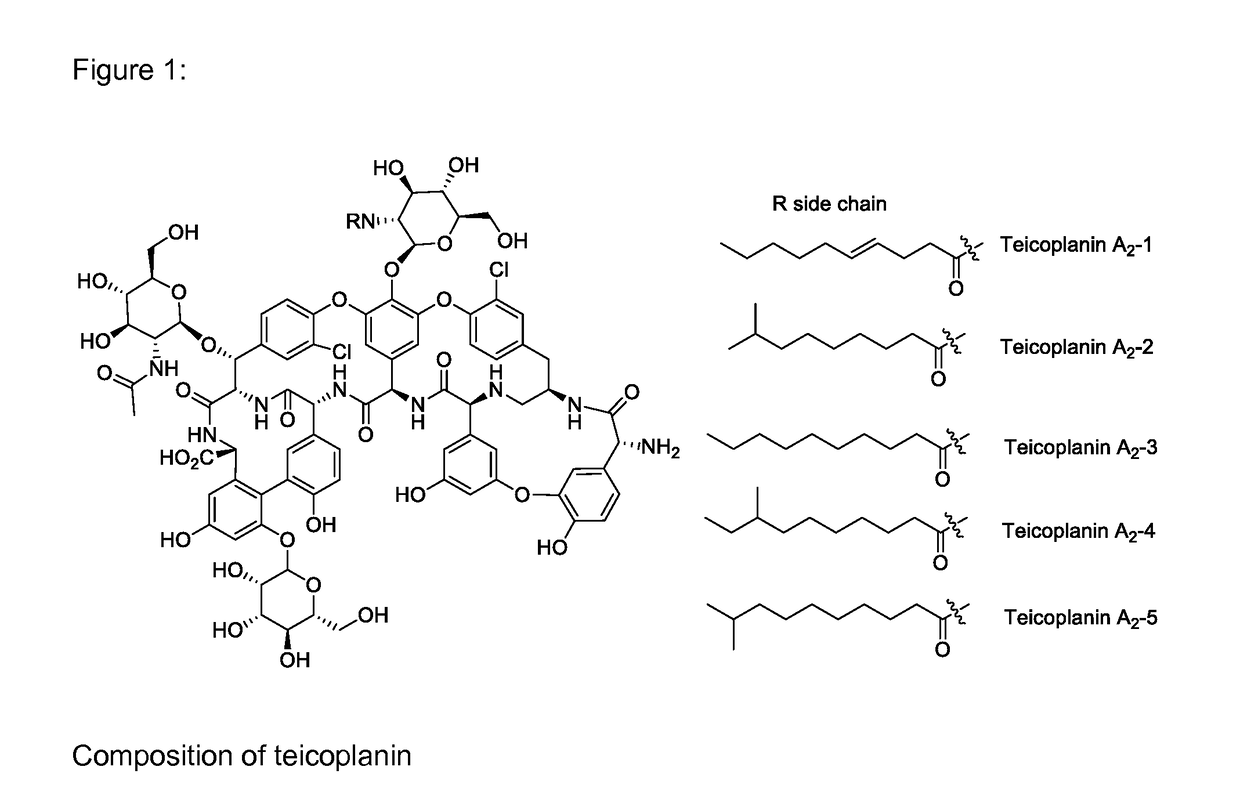
Image
Examples
example 1
Teicoplanin 20 mg / mL Oral Suspension
[0047]
1. Teicoplanin2000mg2. Colloidal silicon dioxide2mg3. Citric acid monohydrate0.3mg4. FD&C Red#30.05mg5. Strawberry flavoring0.05mg6. Propylene glycol15ml7. Water, Purifed15ml8. Sorbitol70ml
Procedure
[0048]1. Calculate the required quantity of each ingredient for the total amount to be prepared.[0049]2. Accurately weigh and / or measure each ingredient.[0050]3. Prepare the suspension using 2 (two) separate Beakers.[0051]4. Beaker 1: levigate teicoplanin (item 1) and colloidal silicon dioxide (item 2) in 30% sorbitol (item 8) (30 ml).[0052]5. Beaker 2: Dissolve citric acid (item 3), strawberry flavor (item 5) and FD&C Red #3 (item 4) in purified water (item 7).[0053]6. Add Beaker 2 to Beaker 1 while stirring then add propylene glycol (item 6) and stir well.[0054]7. Complete to the final volume with sorbitol (item 8) and mix well.
example 2
Teicoplanin 20 mg / mL Oral Suspension
[0055]
1. Teicoplanin2000mg2. Colloidal silicon dioxide2mg3. Sodium Citrate0.3mg4. FD&C Red#30.05mg5. Strawberry flavoring0.05mg6. Propylene glycol15ml7. Water, Purifed15ml8. Sorbitol70ml
Procedure
[0056]1. Calculate the required quantity of each ingredient for the total amount to be prepared.[0057]2. Accurately weigh and / or measure each ingredient.[0058]3. Prepare the suspension using 2 (two) separate Beakers.[0059]4. Beaker 1: levigate teicoplanin (item 1) and colloidal silicon dioxide (item 2) in 30% sorbitol (item 8) (30 ml).[0060]5. Beaker 2: Dissolve sodium Citrate (item 3), strawberry flavor (item 5) and FD&C Red #3 (item 4) in purified water (item 7).[0061]6. Add Beaker 2 to Beaker 1 while stirring then add propylene glycol (item 6) and stir well.[0062]7. Complete to the final volume with sorbitol (item 8) and mix well.
example 3
Teicoplanin 20 mg / mL Oral Suspension
[0063]
1. Teicoplanin2000mg2. Poloxamer 4071mg3. Citric acid monohydrate0.3mg4. FD&C Red#30.05mg5. Strawberry flavoring0.05mg6. Propylene glycol15ml7. Ethanol 95%5ml8. Water, Purifed10ml9. Sorbitol70ml
Procedure
[0064]1. Calculate the required quantity of each ingredient for the total amount to be prepared.[0065]2. Accurately weigh and / or measure each ingredient.[0066]3. Prepare the suspension using 2 (two) separate Beakers.[0067]4. Beaker 1: levigate teicoplanin (item 1) in 30% sorbitol (item 9) (30 ml), then add ethanol (item 7) and stir well.[0068]5. Beaker 2: dissolve citric acid (item 3), poloxamer 407 (item 2), strawberry flavor (item 5) and FD&C Red #3 (item 4) in purified water.[0069]6. Add Beaker 2 to Beaker 1 while stirring then add propylene glycol (item 6) and stir well.[0070]7. Complete to the final volume with sorbitol (item 8) and mix well.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com