Diarylfluorene intermediate preparation method
A technology of diarylfluorene and intermediate, which is applied in the field of preparation of diarylfluorene intermediate material, can solve the problems of complicated separation and purification of intermediate tertiary alcohol, cumbersome preparation of grignard reagent, etc., and achieves the effects of environmental friendliness and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
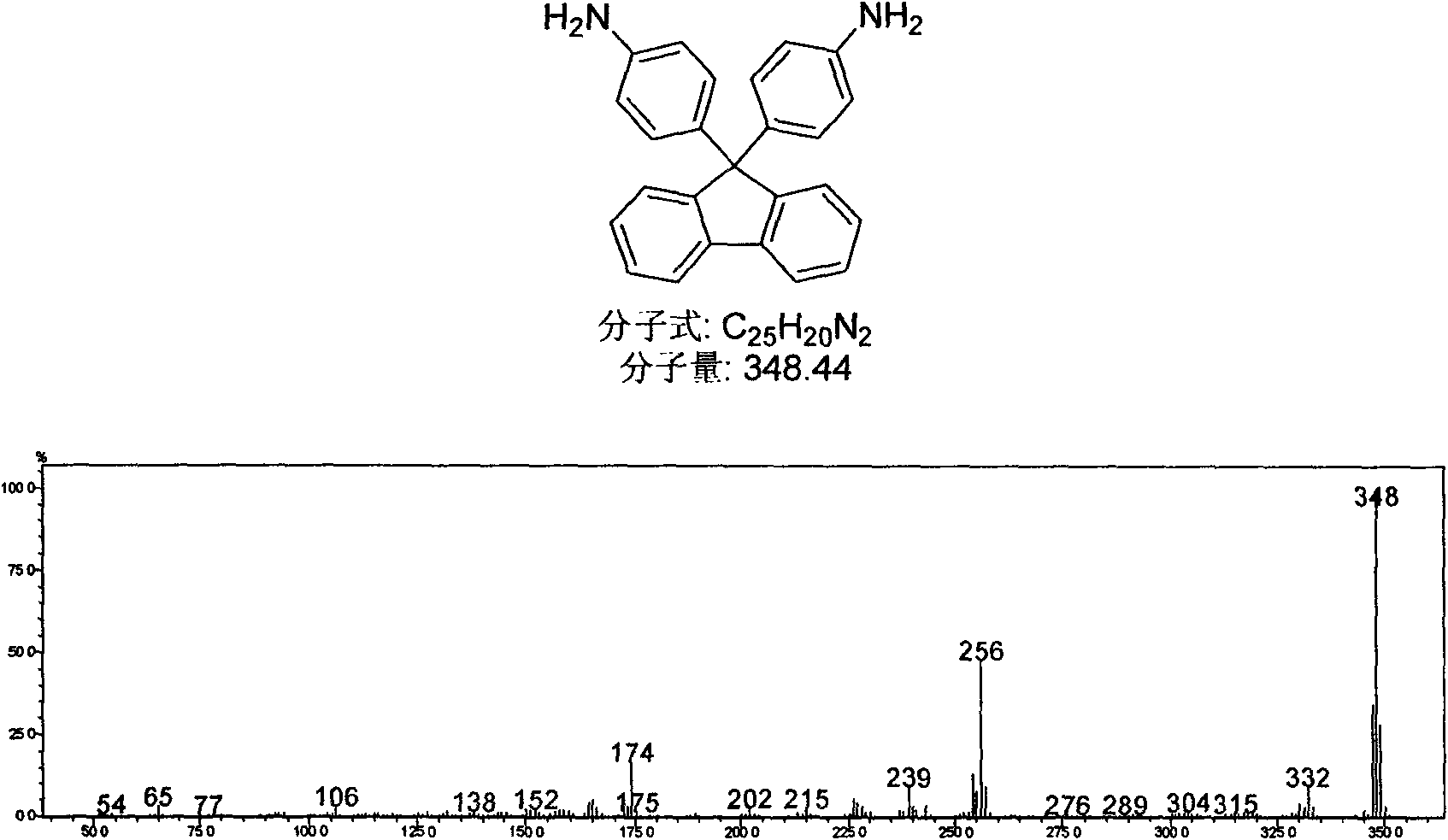
[0032] Embodiment 1:9,9,-the synthesis of two (phenyl) fluorenes
[0033] In a 100mL single-necked flask, add fluorenone (0.9g, 0.005mol, 1eq.), aniline hydrochloride (2.0g, 0.015mol, 3eq.), aniline (10mL), and heat at 150°C for 4 hours under nitrogen protection. After cooling slightly, pour it into 10wt% NaOH solution (50mL), stir for 4h, filter to get pale scarlet fluffy needles, wash with ethanol to remove the pigment, recrystallize with ethanol, and dry in vacuo to obtain a white solid with a melting point of 236-238°C; The air-dried white solid, that is, 9,9'-bis(4-aminophenyl)fluorene (0.348g, 0.001mmol, 1eq.) and 20mL of dichloromethane were added to a 100mL single-necked flask, protected by nitrogen, heated to 40 ° C, 15 Add 0.4mL alkyl nitrite dropwise within 2 minutes, keep reflux for 2h, cool to room temperature, filter off insoluble matter, transfer to a separatory funnel, add 20mL 1M hydrochloric acid, 20mL 10% sodium hydroxide to wash, separate liquid, collect or...
Embodiment 2
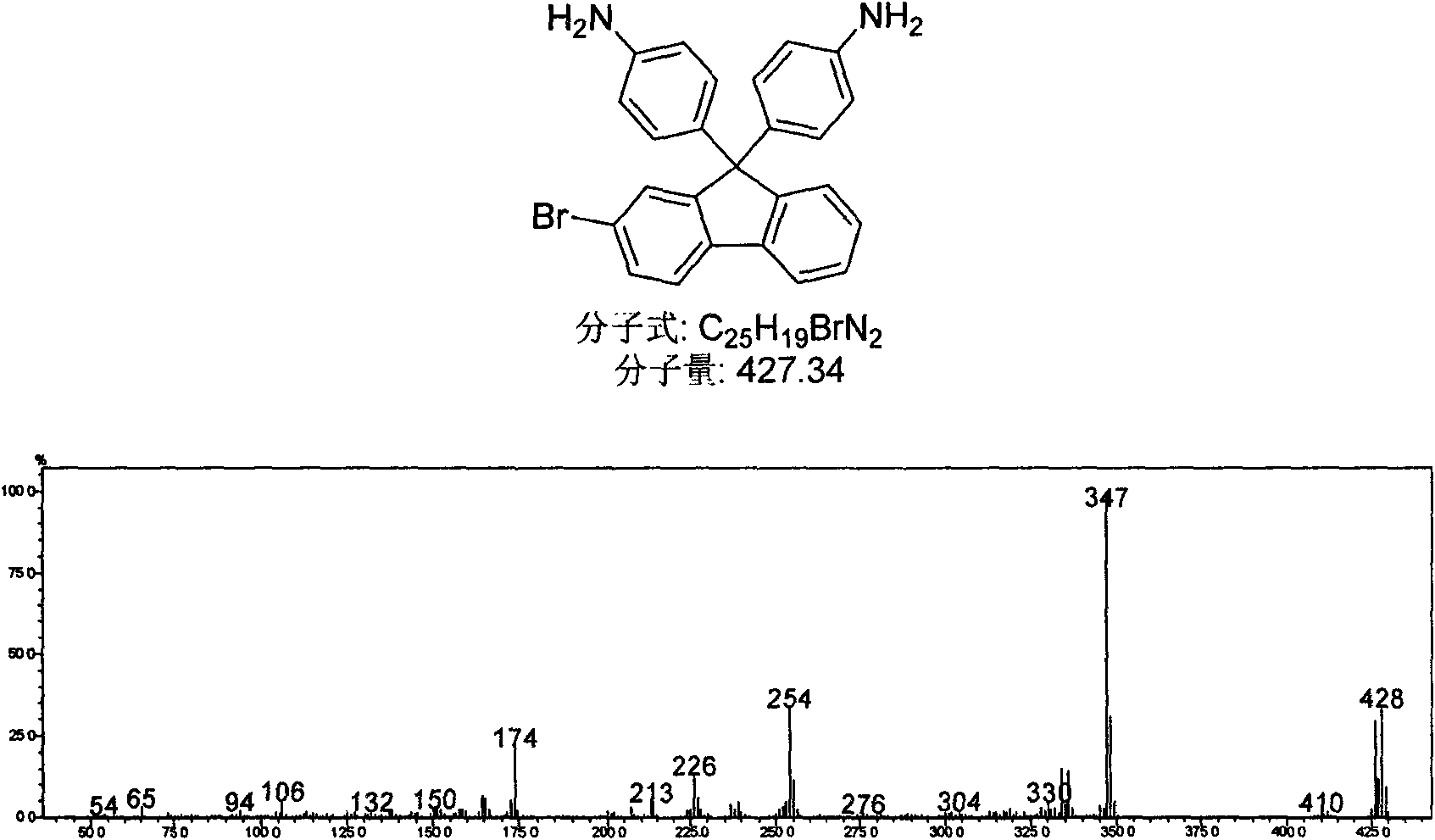
[0034] Example 2: Synthesis of 2-bromo-9,9'-bis(phenyl)fluorene
[0035]In a 100mL single-necked flask, add 2-bromofluorenone (1.30g, 0.005mol, 1eq.), aniline hydrochloride (2.0g, 0.015mol, 3eq.), aniline (10mL), and heat at 150°C under nitrogen protection After 4 hours, cool down slightly and pour into 10wt% NaOH solution (50mL), stir for 4h, filter to get light scarlet fluffy needles, wash with ethanol to remove pigment, recrystallize with ethanol, and dry in vacuo to obtain a white solid, melting point 236-238 ℃; The air-dried white solid is 2-bromo-9,9'-bis(4-aminophenyl)fluorene (0.427g, 0.001mmol, 1eq.) and 20mL of dichloromethane were added to a 100mL single-necked flask, nitrogen protection , heated to 40°C, added dropwise 0.4mL alkyl nitrite within 15 minutes, kept reflux for 2h, cooled to room temperature, filtered off insoluble matter, transferred to a separatory funnel, added 20mL 1M hydrochloric acid, 20mL 10% sodium hydroxide in sequence Wash, separate, collect ...
Embodiment 3
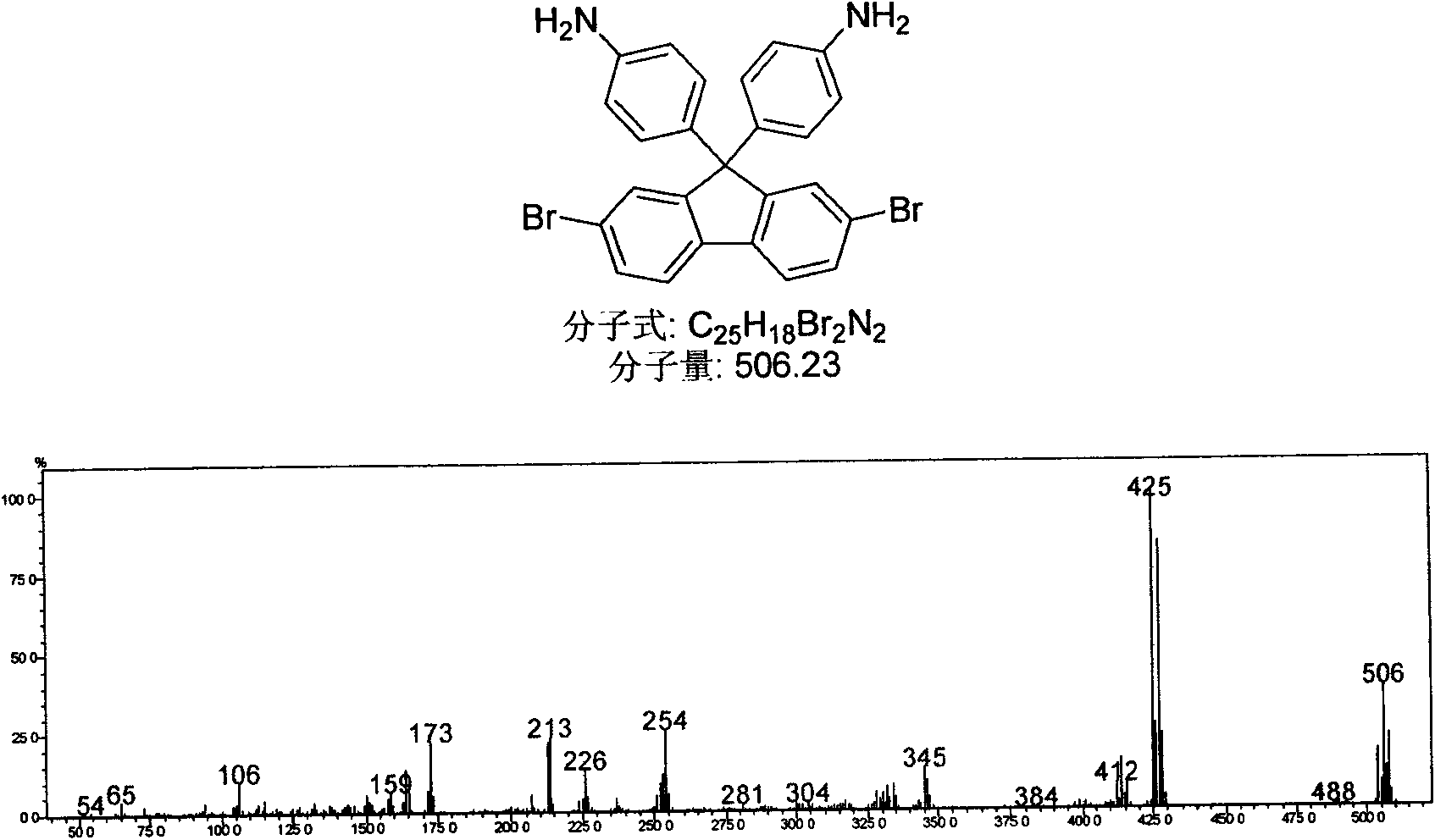
[0037] Embodiment 3: 2,7-dibromo-9,9,-the synthesis of two (phenyl) fluorenes
[0038] In a 100mL single-necked flask, add 2,7-dibromofluorenone (1.68g, 0.005mol, 1eq.), aniline hydrochloride (2.0g, 0.015mol, 3eq.), aniline (10mL), under nitrogen protection Heated at 150°C for 4 hours, cooled slightly, poured into 10wt% NaOH solution (50mL), stirred for 4 hours, filtered to obtain light scarlet fluffy needles, washed with ethanol to remove pigment, recrystallized with ethanol, and dried in vacuo to obtain a white solid, melting point 236-238°C; Air-dried white solid, namely 2,7-dibromo-9,9'-bis(4-aminophenyl)fluorene (0.506g, 0.001mmol, 1eq.) and 20mL of dichloromethane were added to 100mL In a single-necked flask under nitrogen protection, heat to 40°C, add 0.4 mL of alkyl nitrite dropwise within 15 minutes, keep reflux for 2 hours, cool to room temperature, filter out insoluble matter, transfer to a separatory funnel, add 20 mL of 1M hydrochloric acid in sequence, Wash with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com