Double-furofluorenone as well as derivative and preparation method thereof
A technology of difuran and derivatives, applied in the field of new organic field effect transistors, to achieve the effect of broadening the range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0041] The reagents used are all commercially available products, and the solvents are conventionally dried; the reagents used are explained: PE-petroleum ether, DCM-dichloromethane, EA-ethyl acetate, DMF-N.N-dimethylformamide, DEG-dichloromethane Ethylene glycol, N 2 h 4 ·H 2 O-hydrazine hydrate, potassium hydroxide, 1,2-dichloroethane, polyphosphoric acid.
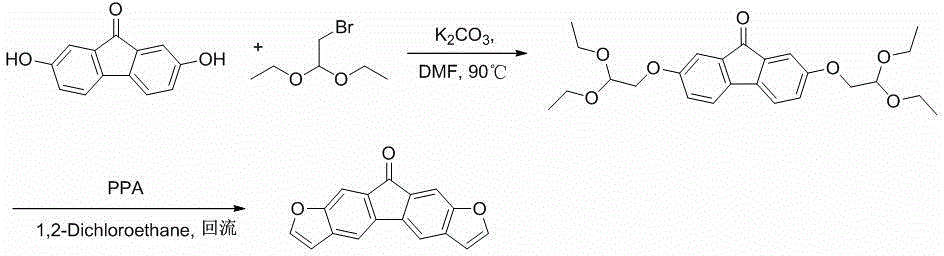
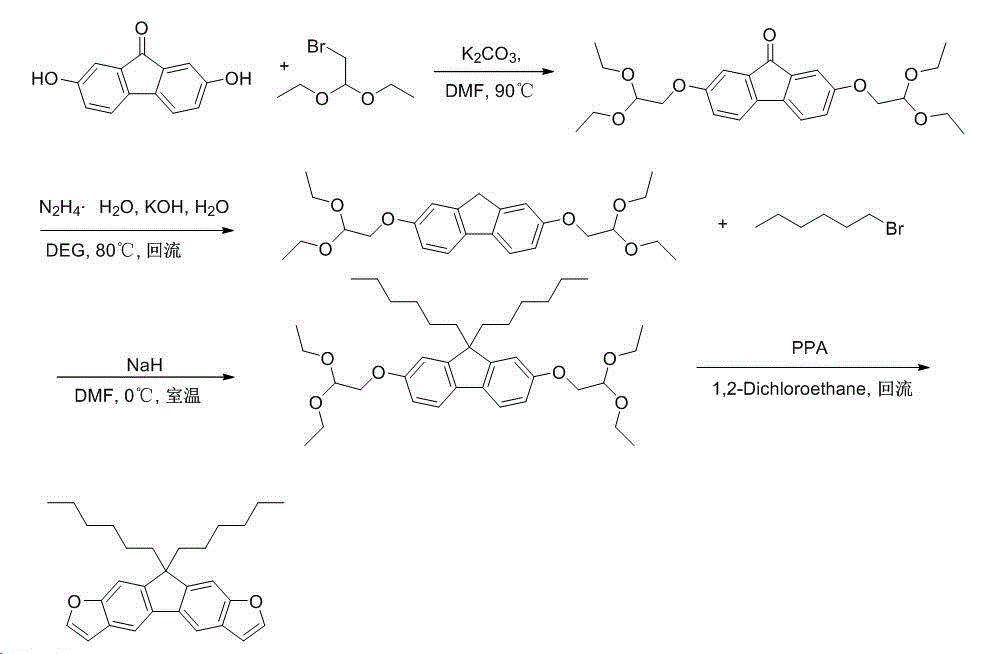
[0042] See attached figure 1 and 2 , the preparation of the compound of the present invention is carried out as follows:
[0043] Preparation of [2,3-b:7,6-b]difurofluorenone (a)
[0044] (1) 2,7-bis(2,2-diethoxyethoxy)-9-fluorenone (1a)
[0045] Dissolve 2.0g (9.43mmol) of 2,7-bis(2,2-diethoxyethoxy)-9-fluorenone in 40mL of DMF, 6.51g (47.25mmol) of potassium carbonate and 4.53ml (28.28mmol ) bromoacetaldehyde diethyl acetal was added to the solution, and the mixture was refluxed for 10 hours; the solution was cooled to room temperature, poured into water to quench the reaction, extracted with dichloromethane (10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com