Crystalline polymorph of fluorene derivative and process for production thereof
A manufacturing method and technology of polymorphs, applied in the field of new crystal polymorphs and their manufacture, capable of solving unknown problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Production of Crude Purified Product
[0067]400 g of toluene and 3.25 g of phosphotungstic acid were added to a glass reactor equipped with a stirrer, a nitrogen blowing tube, a thermometer, and a water separator with a cooling tube, and azeotropic dehydration was carried out under reflux of toluene. Add 129.6 g (0.712 moles) of 9-fluorenone, 994.9 g (7.20 moles) of 2-phenoxyethanol, and 118.7 g toluene, and stir while removing the water generated by the reaction to the outside of the system under toluene reflux. 21 hours. 1,560 g of toluene was added to the obtained reaction mixture, and the obtained mixture was adjusted to 70° C., and washed with 520 g of water four times. The obtained organic layer was concentrated under reduced pressure to remove toluene and excess 2-phenoxyethanol. 1,800 g of toluene was added to the obtained mixture, and 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene was dissolved at 80° C., and the obtained solution was decolorized with...
Embodiment 2
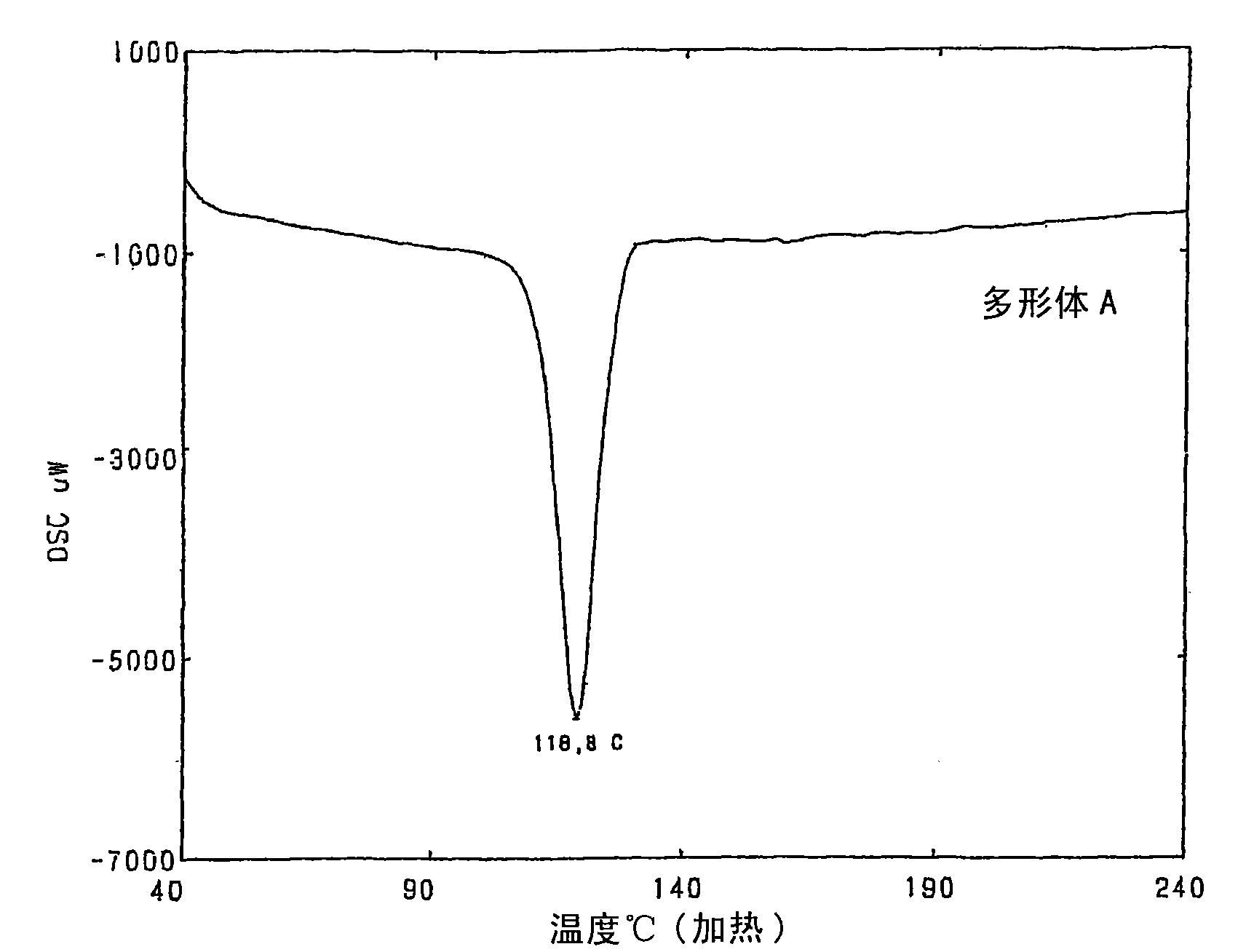
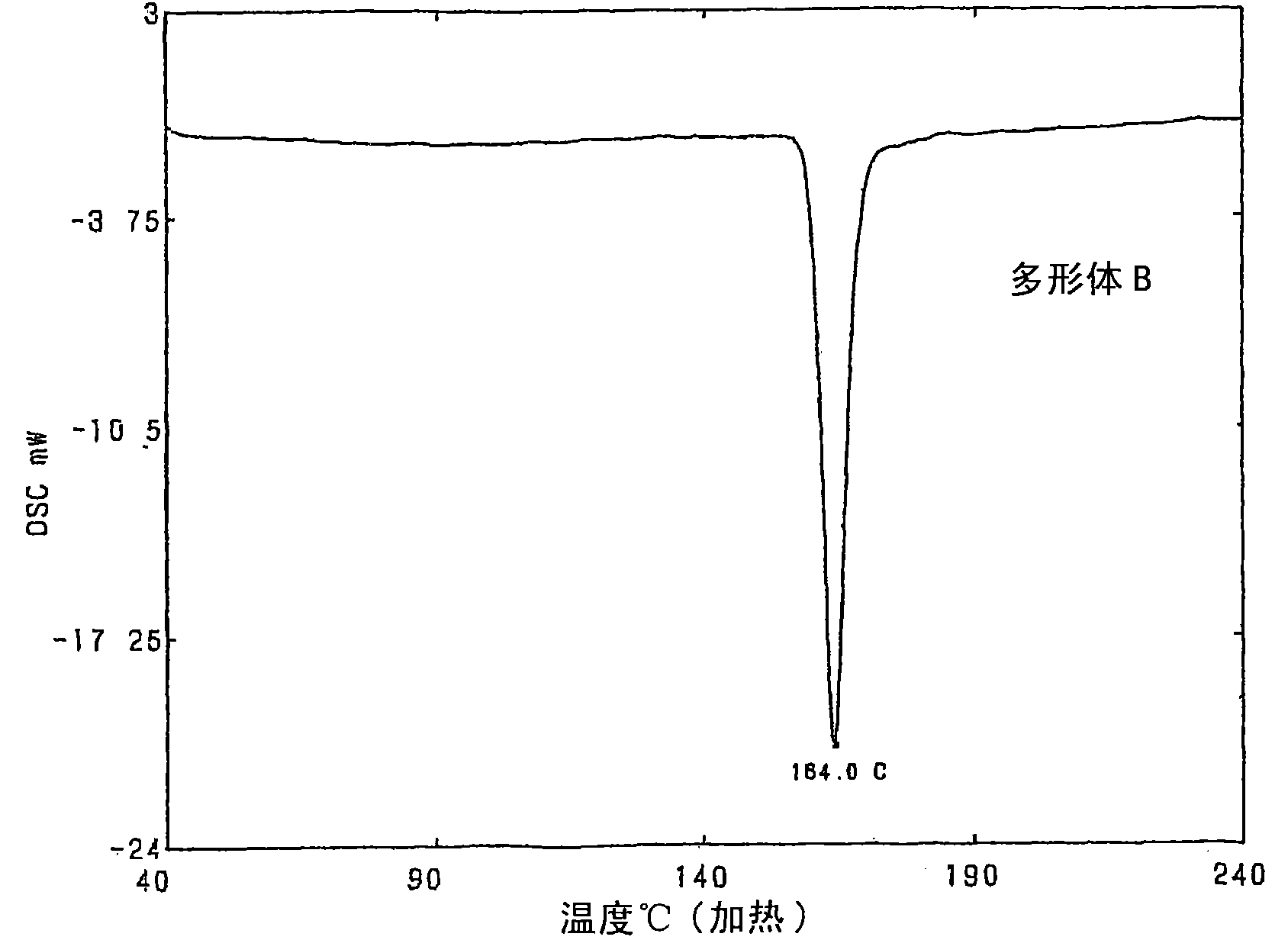
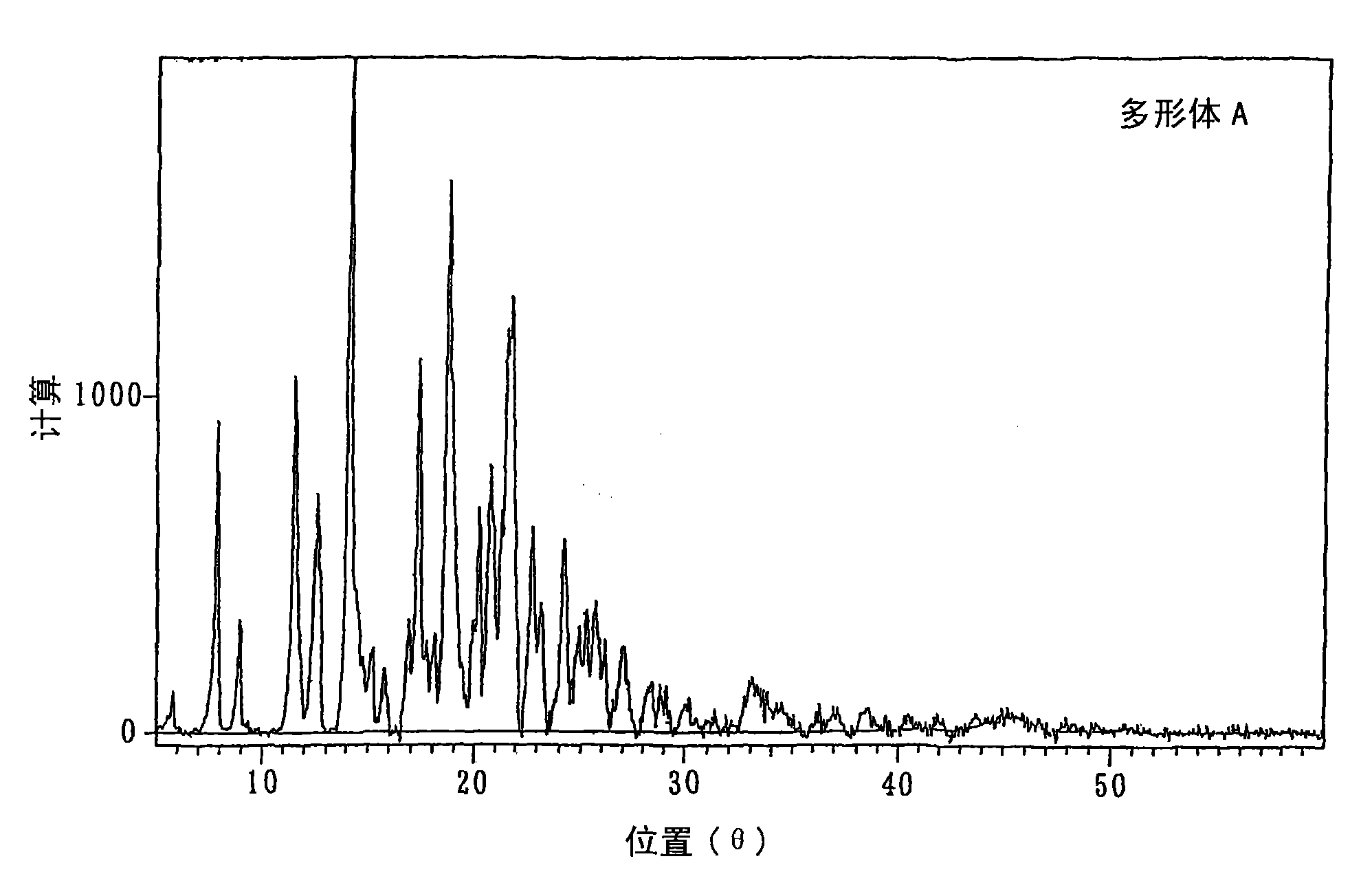
[0069] Manufacture of Polymorph B
[0070] A suspension of 80 g of the crude purified product of 9,9-bis(4-(2-hydroxyethoxy)phenyl) fluorene obtained in Example 1 and 640 g of toluene was heated to 90° C. and stirred at the same temperature for 1 hours to become a homogeneous solution. The solution was cooled to 80°C, 0.4 g of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene (polymorph B) was added as a seed crystal, and stirred at the same temperature for 2 hours to crystallize Precipitate. The liquid was cooled to 20° C. at a cooling rate of 0.2° C. per minute, and stirred at the same temperature for 1 hour to further precipitate crystals. The precipitated crystals were taken out by filtration, and dried under reduced pressure to obtain 73.0 g of white crystals of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene (91.3% yield, 99.2% purity). %). The melting point (maximum melting endothermic peak obtained by differential scanning calorimetry) of the obtained crystals was 164.0° C., a...
Embodiment 3
[0071] Example 3: Production of Crude Purified Product
[0072] 86.4 g (0.48 moles) of 9-fluorenone, 397.9 g (2.88 moles) of phenoxyethanol, 350 g of toluene and Phosphotungstic acid [(H 3 PW 12 o 40 )] 4.3g, under the reflux of toluene, remove the generated water to the outside of the reaction system, and stir for 12 hours at the same time. When the obtained reaction liquid was analyzed by high performance liquid chromatography, 197.3 g (0.45 mol) of 9,9-bis(4-(2-hydroxyethoxy)phenyl)fluorene was produced|generated. To this reaction liquid, 300 g of toluene was added, and 100 g of water was added, followed by washing with water at 80°C. The obtained organic layer was slowly cooled, and as a result, crystals began to precipitate at 12°C, and the mixture was kept cooled down to 10°C, and stirred for 12 hours. The precipitated crystals were taken out by filtration, and the crystals were dried to obtain 158.0 g of white crystals of a crude purified product of 9,9-bis(4-(2-hy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com