Substituted benzophenone and preparation method thereof
A technology of benzophenone and benzophenones, which is applied in the field of compound synthesis, can solve the problems of harsh reaction conditions, limited application of phosgene, complicated operation, etc., and achieves the effects of simple operation and overcoming inability to be widely used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
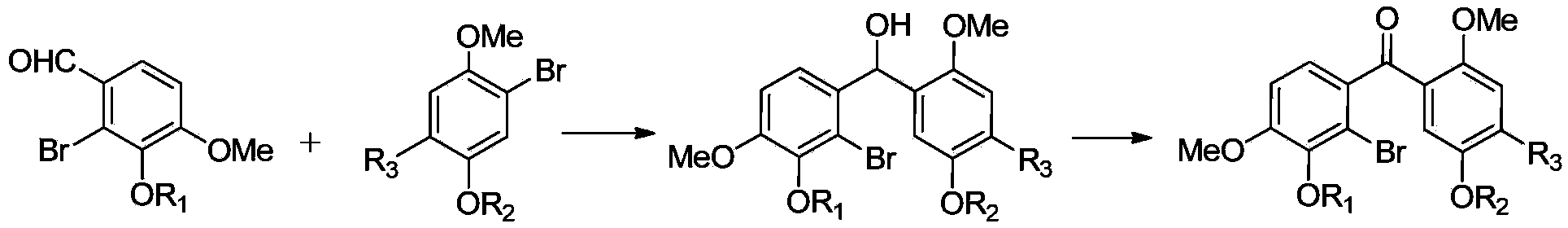
[0042] see figure 1 , the preparation method of above-mentioned substituted benzophenone, comprises the following operations:
[0043] 1) The intermediate compound B is reacted with magnesium in an organic solvent to prepare a Grignard reagent, and then the intermediate compound A is subjected to an addition reaction, and the addition reactant is hydrolyzed to obtain a diphenylcarbinol compound;
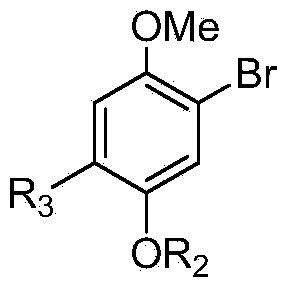
[0044] The structural formula of the intermediate compound B is:
[0045]
[0046] The structural formula of the intermediate compound A is:
[0047]
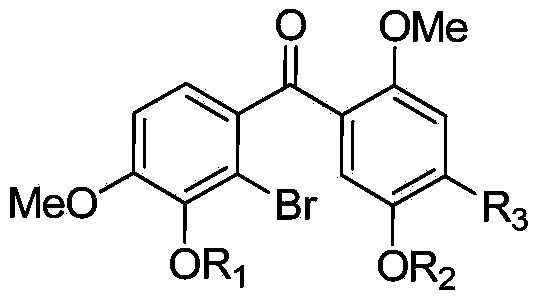
[0048] 2) Oxidizing diaryl carbinol compounds to generate benzophenone compounds to obtain substituted benzophenones with substituents on the benzene rings at both ends of the carbonyl group.
[0049] Specifically, the preparation of the described diphenylcarbinol compound specifically includes the following operations:
[0050] Put anhydrous magnesium chips activated with ammonium chloride solution in a reaction vessel, add ...
Embodiment 1
[0062] Compound I: Synthesis of (5-benzyloxy-2-methoxyphenyl)(2-bromo-3,4-dimethoxyphenyl)methanone, in the structural formula of the compound R 1 , R 2 Respectively methoxy, benzyloxy, R 3 When it is hydrogen, it is prepared by the following steps:
[0063]
[0064] 1) Synthesis of compound (5-benzyloxy-2-methoxyphenyl)(2-bromo-3,4-dimethoxyphenyl)methanol
[0065] Magnesium bars (0.61 g, 0.026 mol) were treated with an aqueous solution of ammonium chloride to remove magnesium oxide from the surface of the bars. Rinse the magnesium bars with tetrahydrofuran to remove water. The treated magnesium strips are placed in a two-neck bottle, and vacuumized to make the surface of the magnesium strips dry. Add two grains of iodine, heat slightly to make it volatilize and adhere to the surface of the magnesium bar, and the magnesium bar is golden yellow. After dissolving 2-bromo-4-benzyloxyanisole (5.0g, 0.017mol) in anhydrous tetrahydrofuran, it was slowly injected into the ab...
Embodiment 2
[0069] Compound Ⅱ: Synthesis of (3-benzyloxy-2-bromo-4-methoxyphenyl)(5-benzyloxy-2-methoxyphenyl)methanone
[0070]
[0071] 1) Synthesis of (5-benzyloxy-2-methoxyphenyl)(3-benzyloxy-4-methoxyphenyl)methanone
[0072] Magnesium chips (0.62 g, 0.025 mol) were activated with saturated ammonium chloride solution until bright, filtered with suction, washed with a large amount of water first, and washed with anhydrous tetrahydrofuran immediately after changing the filter paper until anhydrous. After adding to the three-necked bottle, evacuate three times with an oil pump under heating conditions, and dry and store under a nitrogen atmosphere. After adding a grain of iodine to the system, evacuate and fill with nitrogen three times. First add a small amount of 2-bromo-4-benzyloxyanisole (5.00g, 0.017mol) in anhydrous tetrahydrofuran solution through a syringe, and initiate the reaction under heating conditions (the solution changes from light yellow to taupe), and then the rema...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com