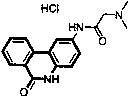
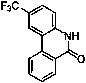
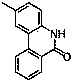
Environment-friendly synthetic method of 6(5H)-phenanthridine derivative
A technology of green synthesis and derivatives, applied in the field of synthesis of pharmaceutical and chemical intermediates, can solve the problems of unavailable starting materials, difficult derivation, and complicated experimental operations, and achieve high reaction chemical selectivity and regioselectivity, and great implementation value and social and economic benefits, and the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of phenanthridine-6(5H)-one (Ⅲa)
[0022] Will N -(1,1'-biphenyl)-2-pyridineamide (0.82g, 3mmol), diisopropyl azodicarboxylate (1.21g, 6mmol), cobalt acetate tetrahydrate (0.11g, 0.45mmol), A solution of sodium pivalate (0.85g, 6mmol) and oxygen (1 atm) in dioxane (8mL) was stirred at 80°C for 24 hours and separated by silica gel column chromatography to obtain a white solid phenanthridine-6(5H) - Ketone (Ⅲa) 0.36g, yield: 62%, melting point: 297.5~298.3°C. The structural formula of Ⅲa is:
[0023]
[0024] 1 H NMR (600 MHz, DMSO- d 6 , ppm) δ 11.70 (s, 1H), 8.50 (d, J = 8.4 Hz, H),8.38 (d, J = 7.8 Hz, 1H), 8.34 (d, J = 7.8 Hz, 1H), 7.86 – 7.83 (m,1H), 7.66– 7.63 (m, 1H), 7.50 – 7.47 (m, 1H) , 7.39 (d, J = 7.8 Hz, 1H), 7.27 – 7.24(m, 1H). 13 C NMR (150 MHz, DMSO- d 6 , ppm) δ 161.3, 137.0, 134.7, 133.2, 130.0,128.4, 127.9, 126.2, 123.7, 123.1, 122.7, 118.0, 116.6. 13 h 8 NO [M-H] - : 194.0611; Found: 194.0610.
Embodiment 2
[0025] Example 2: Preparation of phenanthridine-6(5H)-one (Ⅲa)
[0026] Will N -(1,1'-biphenyl)-2-pyridineamide (0.82g, 3mmol), diisopropyl azodicarboxylate (1.21g, 6mmol), cobalt acetate tetrahydrate (0.11g, 0.45mmol), A solution of sodium pivalate (0.85g, 6mmol) and oxygen (1 atm) in dioxane (8mL) was stirred at 150°C for 24 hours and separated by silica gel column chromatography to obtain a white solid phenanthridine-6(5H) - Ketone (Ⅲa) 0.47g, yield: 80%.
Embodiment 3
[0027] Embodiment 3: Preparation of phenanthridine-6(5H)-one (Ⅲa)
[0028] Will N-(1,1'-biphenyl)-2-pyridineamide (0.82g, 3mmol), diisopropyl azodicarboxylate (1.21g, 6mmol), cobalt chloride (0.06g, 0.45mmol), Tetra A solution of sodium valerate (0.85g, 6mmol) and oxygen (1 atm) in dioxane (8mL) was stirred and reacted at 120°C for 10 hours. After the reaction, it was separated by silica gel column chromatography to obtain a white solid phenanthridine-6 (5H)-ketone (Ⅲa) 0.44g, yield: 75%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com