Synthesis and application of dinuclear ring metal platinum (II) complex near-infrared electrophosphorescent material containing different conjugated bridges
A technology of cyclometals and complexes, which is applied in the direction of luminescent materials, compounds containing elements of group 8/9/10/18 of the periodic table, platinum group organic compounds, etc. Weak effect, affecting luminous efficiency and other issues, to achieve the effect of enhancing external quantum efficiency, broadening energy level delocalization, and reducing non-radiative transition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Cyclometallic Platinum Complex IQBIQ[Pt(DPM)] 2 、IQDFBIQ[Pt(DPM)] 2 and PyrBIQ[Pt(DPM)] 2 The preparation route of is as follows.
[0056]
[0057] (1) Synthesis of intermediate 1
[0058] Add compound 1,4-dibromobenzene (5g, 21.37mmol), bipinacol borate (13.56g, 53.42mmol), potassium acetate (12.58g, 128.22mmol), Pd(dppf ) Cl 2 (781mg, 1.069mmol) and 1,4-dioxane (40mL), under the protection of nitrogen, the temperature was raised to 80°C and the reaction was refluxed for 24h. The reaction was stopped, cooled, the reaction solution was washed 4 times with saturated brine, extracted 2 times with DCM, the combined organic phases were washed with anhydrous MgSO 4 Dry overnight, filter, and spin off the organic solvent. The crude product is separated by column chromatography using PE-DCM (v / v, 2 / 1) as the eluent, and then recrystallized from n-hexane to obtain 4.8 g of white needle-like crystals. Yield: 68.5%. 1 H NMR (400MHz, CDCl 3 ,TMS),δ(ppm):7.80(s,1H),1.35(...
Embodiment 2
[0079] Structural characterization of near-infrared electrophosphorescent materials of dinuclear cyclometal platinum(II) complexes with different conjugated bridges, and their photoelectric physical, electrochemical, thermodynamic properties and luminescence performance tests.
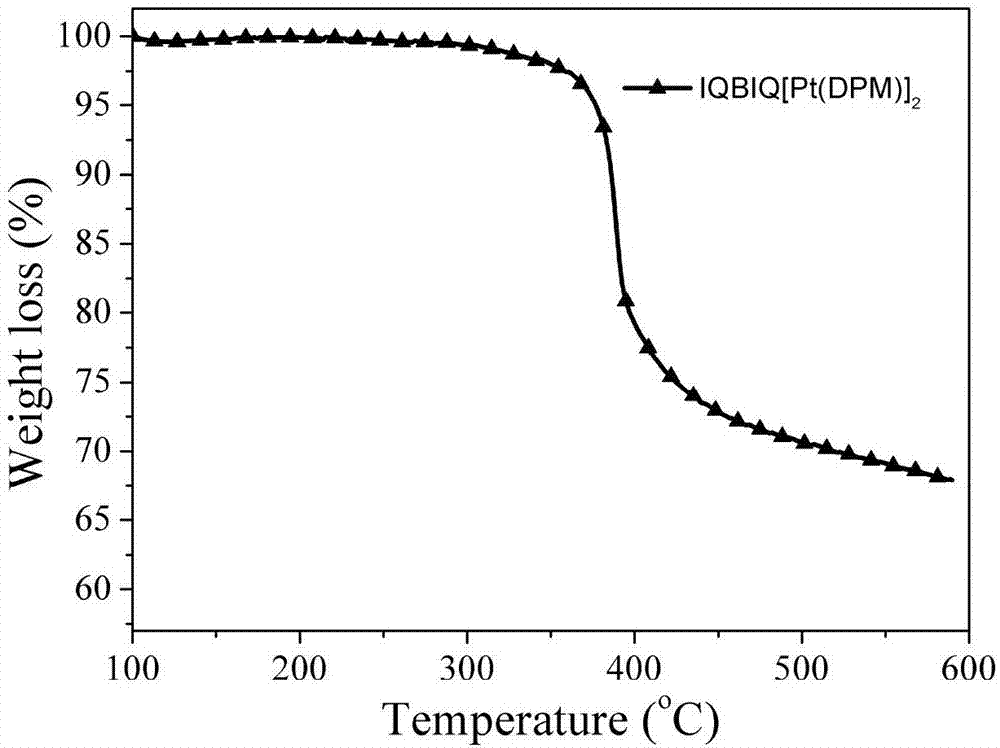
[0080] Symmetrical Binuclear Cyclometallic Platinum(II) Complexes with A-D-A Configuration 1 H NMR, 13 C NMR spectrum was measured by BrukerDex-400NMR instrument; mass spectrum was tested by Bruker Bifiex MALDI-TOF mass spectrometer; thermogravimetric analysis was carried out by thermal analyzer model Perking-El TGA at 10 °C min -1 Tested at the heating rate; cyclic voltammetry (CV) adopts the electrochemical workstation model HP-CHI660A, under the protection of nitrogen, with a three-electrode system test (Pt sheet as the working electrode, Pt wire as the reference electrode, Ag / AgCl For the reference electrode, 0.1M Bu 4 NPF 6 Acetonitrile solution is electrolyte); UV-visible absorption spectrum i...
Embodiment 3
[0093] Photophysical and Thermodynamic Properties of Symmetrical Binuclear Cyclometallic Platinum Complexes with Different Conjugated Bridges
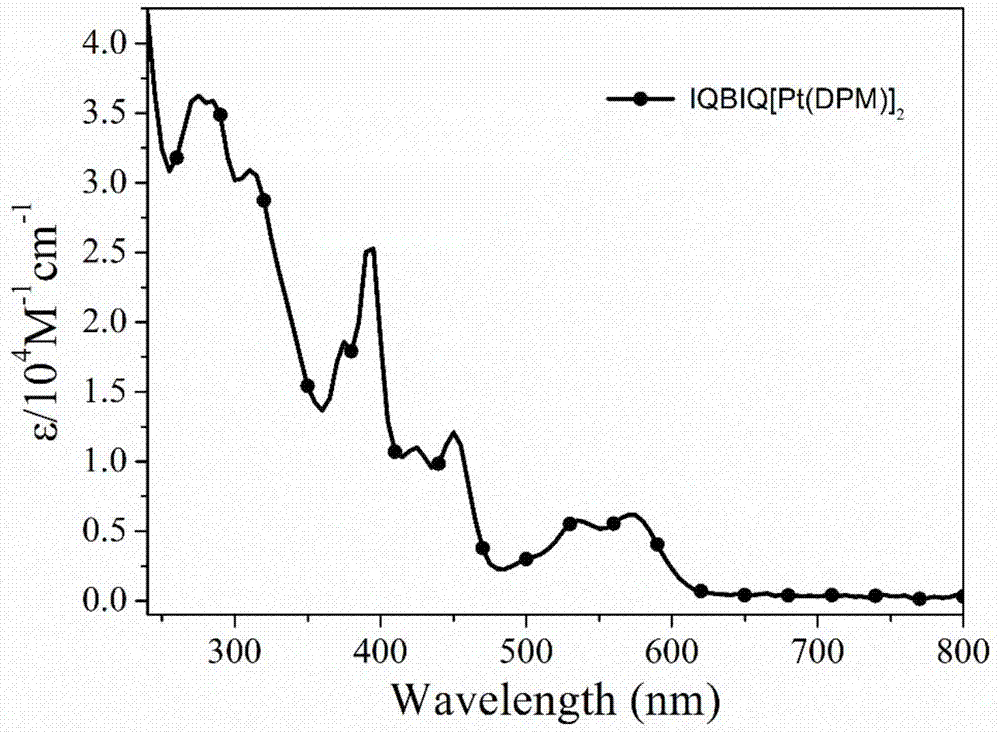
[0094] figure 1 , Figure 6 and Figure 11 They are binuclear cyclometal platinum complexes IQBIQ[Pt(DPM)] 2 、IQBIQ[Pt(DPM)] 2 and PyrBIQ[Pt(DPM)] 2 The ultraviolet-visible (UV-vis) absorption spectrogram in DCM solution, specific data are shown in Table 1. The strong absorption band located in the 240-390nm interval belongs to the spin-allowed π-π* transition centered on the main ligand (LC), and the low-energy band absorption peak at 350-600nm belongs to the ligand-centered spin-forbidden ( 3 LC) electron transition, metal to ligand ( 3 MLCT) charge transfer.
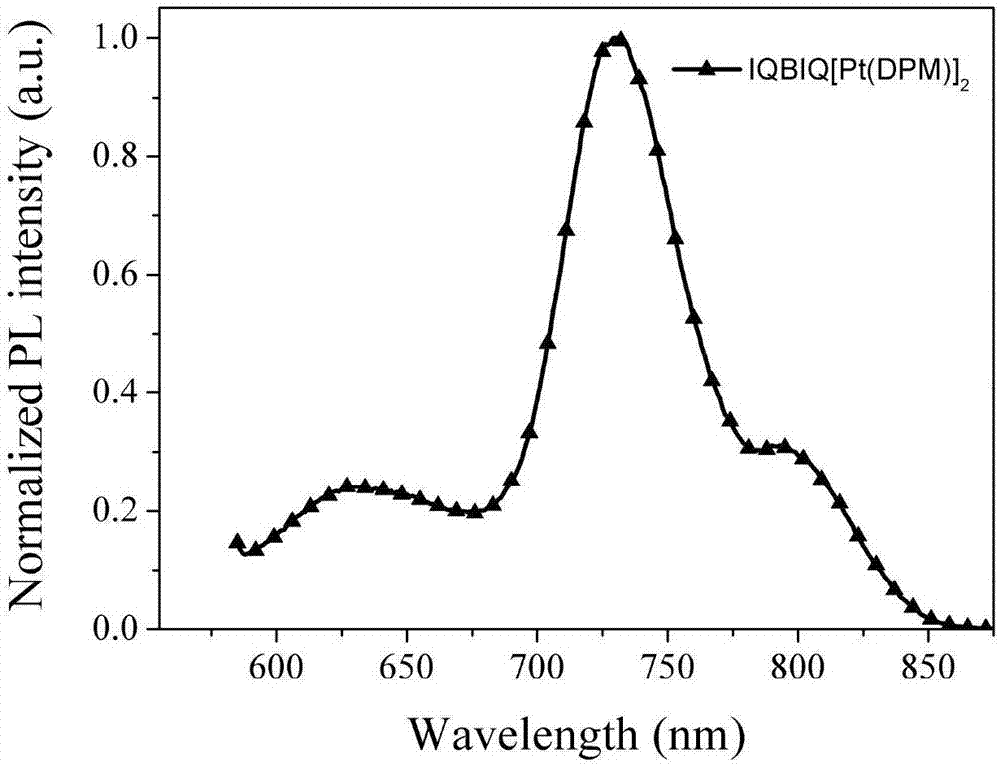
[0095] figure 2 , Figure 7 and Figure 12 They are binuclear cyclometal platinum complexes IQBIQ[Pt(DPM)] 2 、IQBIQ[Pt(DPM)] 2 and PyrBIQ[Pt(DPM)] 2 The photoluminescence spectrum in DCM solution, the specific data are shown in Table 1. The study found that IQBI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com