Benzodithiophene copolymer containing isoindoline-1,3-dione unit and its preparation method and application
A technology of benzodithiophene and copolymers, which is applied in chemical instruments and methods, luminescent materials, organic chemistry, etc., and can solve the problem of low photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
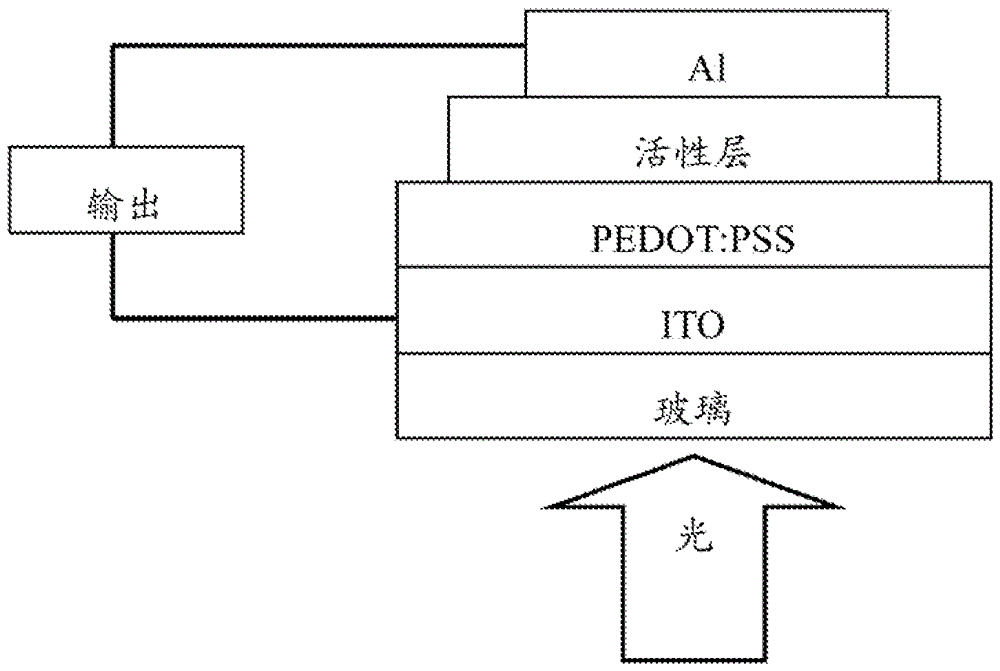
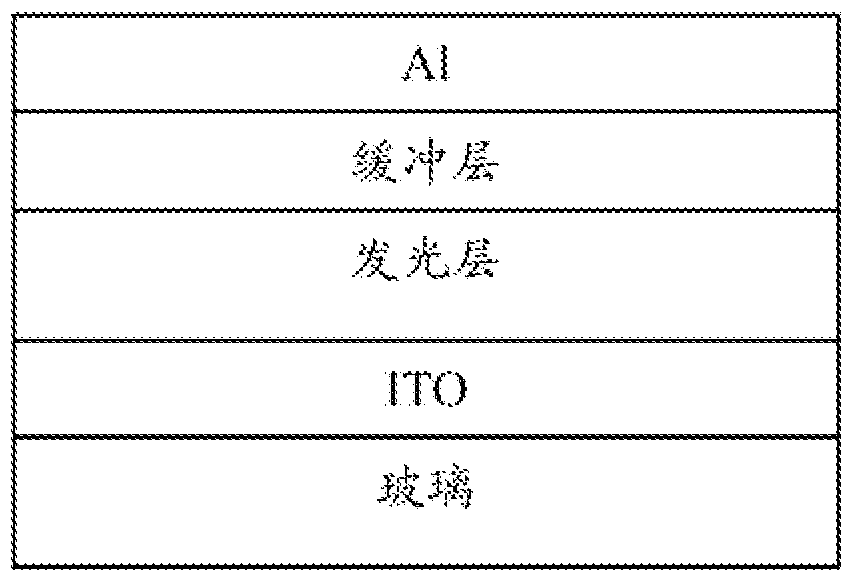
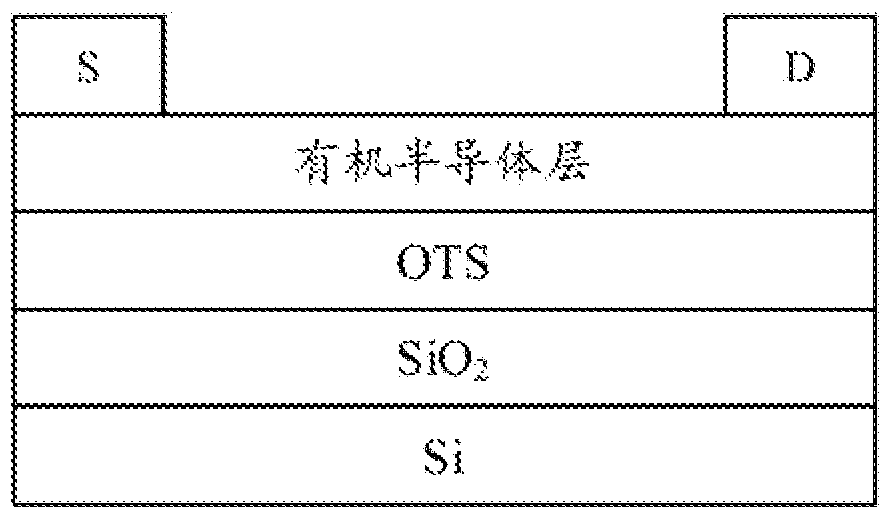
Image
Examples
Embodiment 1
[0083] Embodiment 1. This embodiment discloses a benzodithiophene copolymer containing isoindoline-1,3-dione units with the following structure:
[0084]
[0085] Among them, R 1 , R 2 Same, both H; R 3 is octyloxy; R 4 for H; R 5 It is 16 alkyl groups, as shown in the figure, it is 2-hexyldecyl; n=60.
[0086] The preparation steps of the above-mentioned benzodithiophene copolymers containing isoindoline-1,3-dione units are as follows:
[0087] One, the preparation of 1,3-two (2-thiophene) acetone:
[0088]
[0089] First, dissolve 7.6 g, 36.8 mmol of DCC, 1.23 g, 10 mmol of DMAP in 70 mL of anhydrous dichloromethane, and dissolve 5 g, 35.2 mmol of 2-thiopheneacetic acid in 70 mL of dichloromethane under nitrogen protection. The methane solution was added dropwise to the above reaction solution and reacted overnight. After the reaction, the reaction solution was filtered, recrystallized twice with n-hexane, and then separated and purified by column chromatography...
Embodiment 2
[0110] Example 2. This example discloses a benzodithiophene copolymer containing isoindoline-1,3-dione units with the following structure:
[0111]
[0112] Among them, R 1 is hexyl; R 2 for H; R 3 For 2-methylthienyl; R 4 for H; R 5 is octyl, such as 1-n-octyl; n=55.
[0113] The above-mentioned preparation steps of the benzodithiophene copolymer containing isoindoline-1,3-diketone unit are as follows:
[0114] One, the preparation of two (4-dihexyl-2-thiophene) acetone:
[0115]
[0116] First, dissolve 7.6g of DCC and 1.23g of DMAP in 70mL of anhydrous dichloromethane, and add 7.6g of 4-hexyl-2-thiopheneacetic acid in 60mL of dichloromethane dropwise to the above-mentioned reaction solution overnight. After the reaction, the reaction solution was filtered, recrystallized twice with n-hexane, and then separated and purified by column chromatography to obtain the product.
[0117] MALDI-TOF-MS (m / z): 391 (M + ).
[0118] Two, 2,7-two (2-methyl-5-thiophene) ben...
Embodiment 3
[0134] Example 3. This example discloses a benzodithiophene copolymer containing isoindoline-1,3-dione units with the following structure:
[0135]
[0136] Among them, R 1 is 16 alkyl; R 2 for H; R 3 is methoxy; R 4 for H; R 5 is methyl; n=35.
[0137] 1. 2,5-dimethoxybenzene-7,9-bis(4-hexadecyl-5-trimethyltin-2-thiophene)-8H-cyclopentadienebenzo[1,2- b: Preparation of 4,3-b']dithiophen-8-one:
[0138]
[0139] For the synthesis method, refer to Step 1 to Step 4 in Example 1 above.
[0140] MALDI-TOF-MS (m / z): 1241 (M+ ).
[0141] Two, the preparation of the benzodithiophene copolymer containing isoindoline-1,3-dione unit:
[0142]
[0143] Under nitrogen protection, 0.74 g, 0.6 mmol of 2,5-dimethoxybenzene-7,9-bis(4-hexadecyl-5-trimethyltin-2-thiophene)-8H-cyclo Pentadienebenzo[1,2-b:4.3-b']dithiophen-8-one and 0.16g, 0.5mmol of 4,7-dibromo-2-methylisoindoline-1,3- Diketone was added to a reaction flask filled with 10 mL of dry DMF, and the reaction mixtur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com