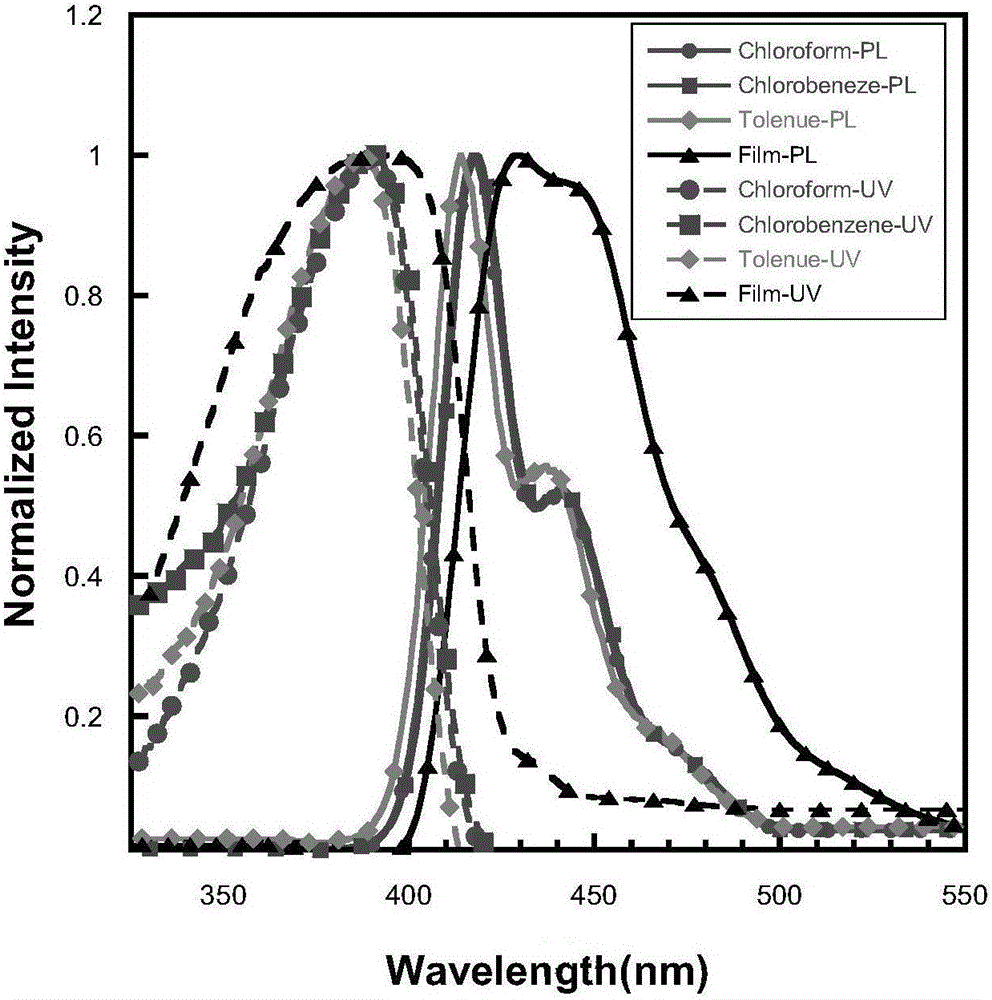
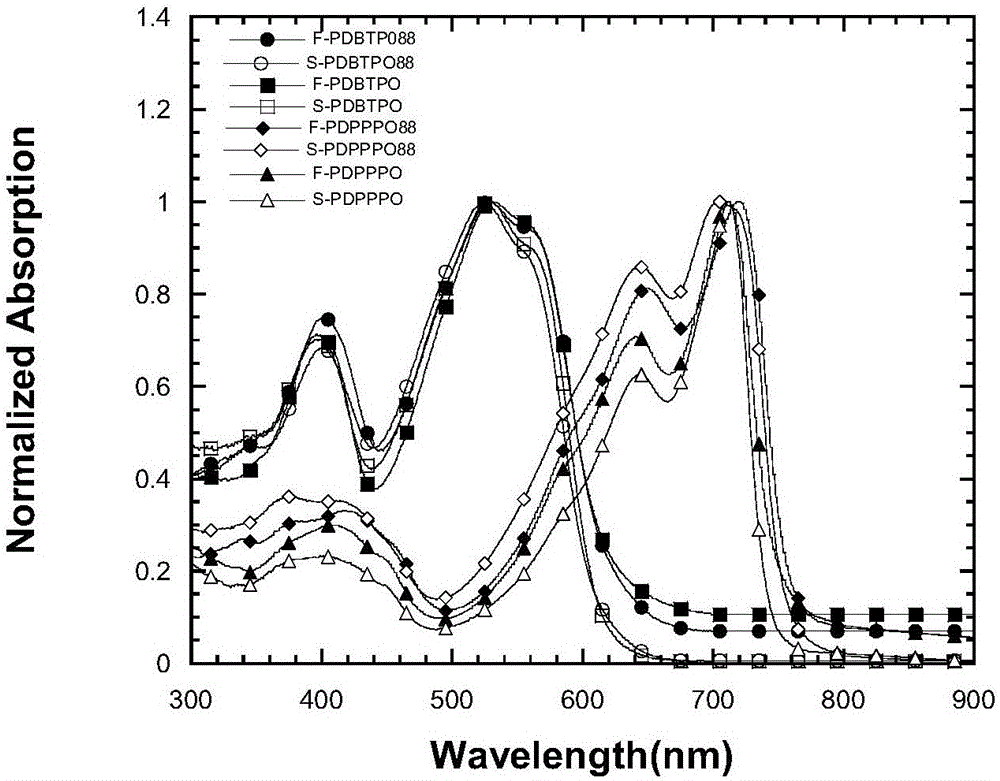
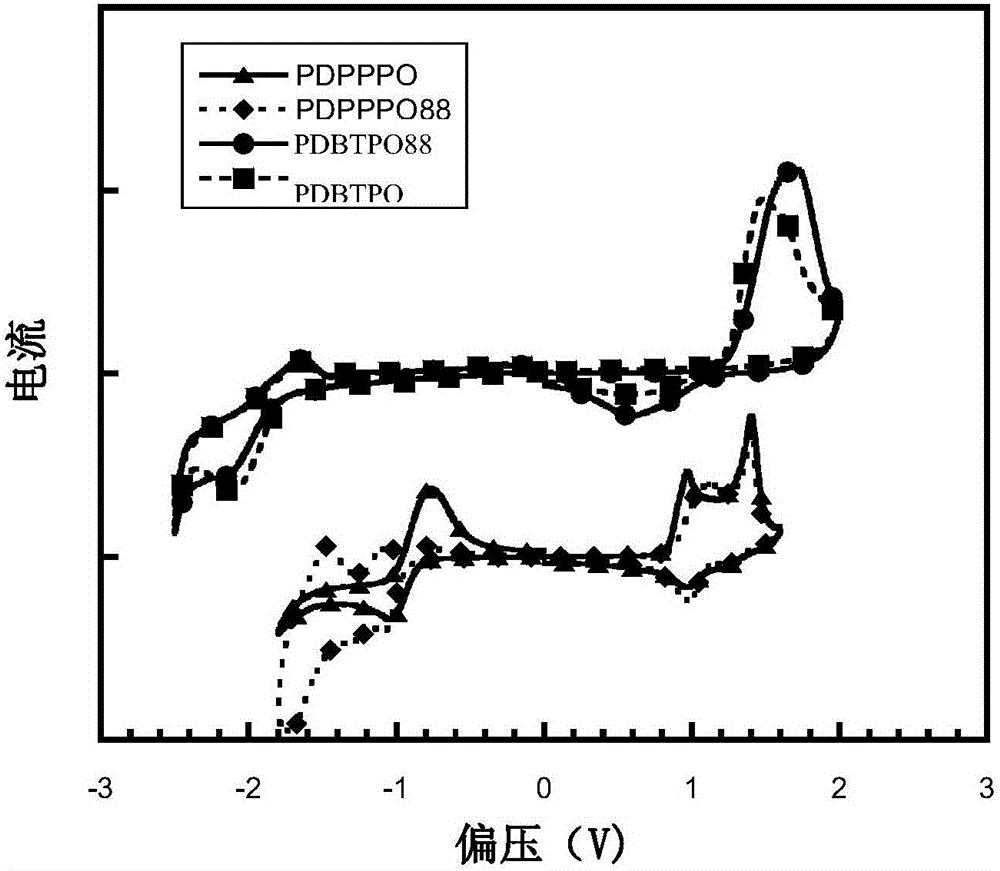
Organic semiconductor materials containing 2,9-dialkyl-6-alkoxy phenanthridine unit and preparation method and application thereof
An organic semiconductor, alkoxyphenanthridine technology, applied in the field of organic optoelectronic materials, can solve the problems of insufficient blue spectrum, narrow polymer energy band, etc., and achieve the effects of easy purification, improved open circuit voltage, and improved color purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1, 3,8-dibromo-6-((2-octyldodecyl)oxy)phenanthridine (abbreviated as Br 2 -PO) synthesis
[0049] The chemical reaction process is shown in the following formula, and the specific reaction steps and reaction conditions are as follows:
[0050]
[0051] Among them, (I) chromium trioxide, glacial acetic acid, 60°C (II) sodium azide, concentrated sulfuric acid, ice bath for 4 hours; (III) 2-octyl dodecyl bromide, potassium carbonate, DMF, reflux for 12 hours.
[0052] (1) Dissolve 2,7-dibromofluorene (19.5g, 60mmol) in 400ml of glacial acetic acid and stir, add chromium trioxide (18.1g, 180mmol) and heat to 60°C, react overnight, pour the reaction the next day into crushed ice, filtered and washed the filter cake with water three times, and dried the filter cake to obtain a yellow solid (compound 1, 19.1 g, yield 95%). The raw material 2,7‐dibromofluorene was ordered from Aldrich Company.
[0053] (2) Dissolve 2,7-dibromofluorenone (compound 1, 2.36g, 7mmol) ...
Embodiment 2
[0055] Example 2, 3,8-dibromo-2,9-dioctyl-6-((2-octyldodecyl)oxy)phenanthridine (abbreviated as Br 2 -PO88) synthesis
[0056]
[0057] Among them, (I) ethylene glycol, trimethyl orthoformate, toluene, p-toluenesulfonic acid, reflux; (II) n-octane bromide, magnesium chips, tetrahydrofuran, Ni(dppf)Cl 2 , heated to reflux for two days, 2M HCl; (Ⅲ) liquid bromine, iron powder, chloroform; (Ⅳ) sodium azide, concentrated sulfuric acid; (Ⅴ) 2-octyl dodecyl bromide, potassium carbonate, DMF, reflux 12 hours.
[0058] (1) Order 3,6-dibromofluorene raw material fluorene from Aldrich Company.
[0059] (2) Synthesis of compound 4[3,6-dibromospiro[fluorene-9,2'-[1,3]dioxolane]
[0060] Compound 3 (10 g, 30 mmol), 8 ml of ethylene glycol, and 0.05 g of p-toluenesulfonic acid were dissolved in 200 ml of toluene, and 9 g of trimethyl orthoformate was added after argon gas flowed for 10 min, and heated to reflux for one day. The next day, the reaction solution was washed three times w...
Embodiment 3
[0069] The synthesis of embodiment 3, polymer PDBTPO
[0070] The polymerization reaction process is shown in the following formula, and the specific steps and reaction conditions are as follows:
[0071]
[0072] Among them, (I) chlorobenzene, Pd 2 (dba) 3 , P(o-tol) 3 , microwave reaction, 140°C, 45 minutes.
[0073] Weigh monomer Br 2 -PO (61.3mg, 0.1mmol), monomer DBT (88.2mg, 0.1mmol) were placed in a microwave reaction tube, chlorobenzene (3mL) was added, and argon was passed for 20 minutes. Then add the catalyst Pd 2 (dba) 3 (3mg) and ligand P(o-tol) 3 (7 mg), argon gas was passed through the reaction tube to fill the reaction tube with argon gas, the lid was closed, microwave reaction was carried out, and the reaction was carried out at 140° C. for 45 minutes. After the reaction is over, drop the reaction solution into methanol to precipitate the polymer, then wash the polymer with methanol, acetone, and n-hexane in a Soxhlet extractor, filter the head, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com