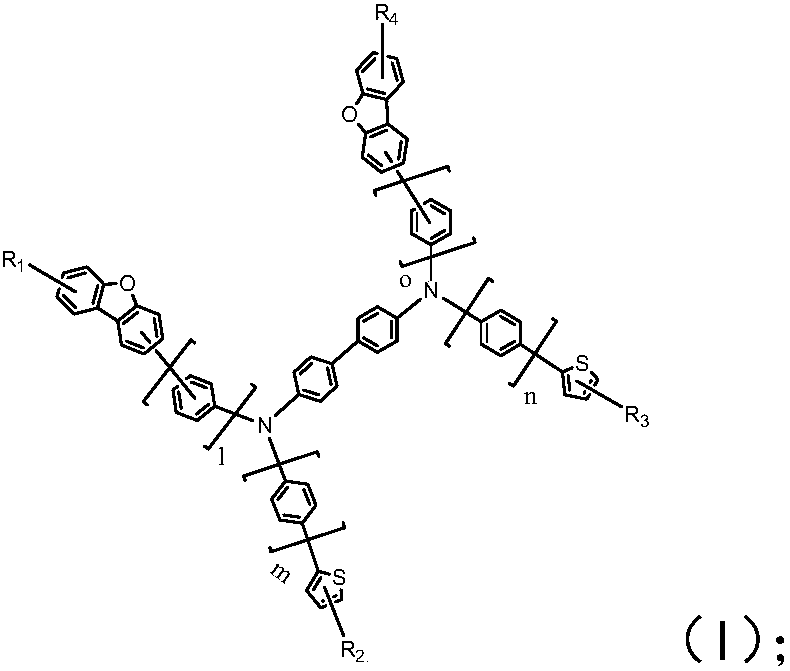
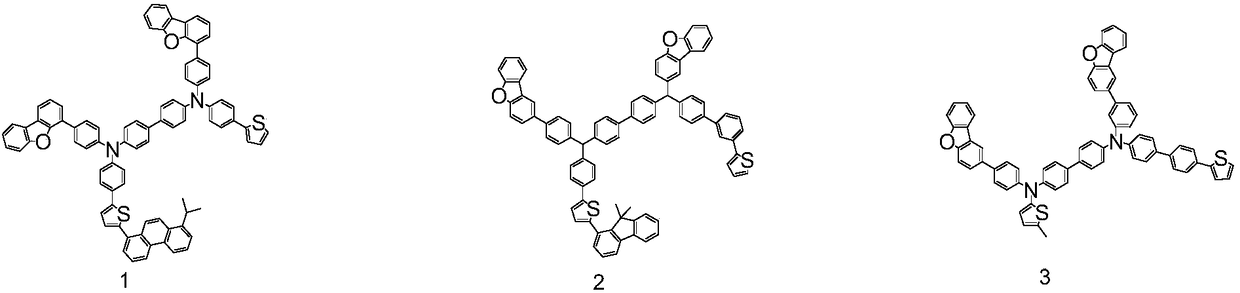
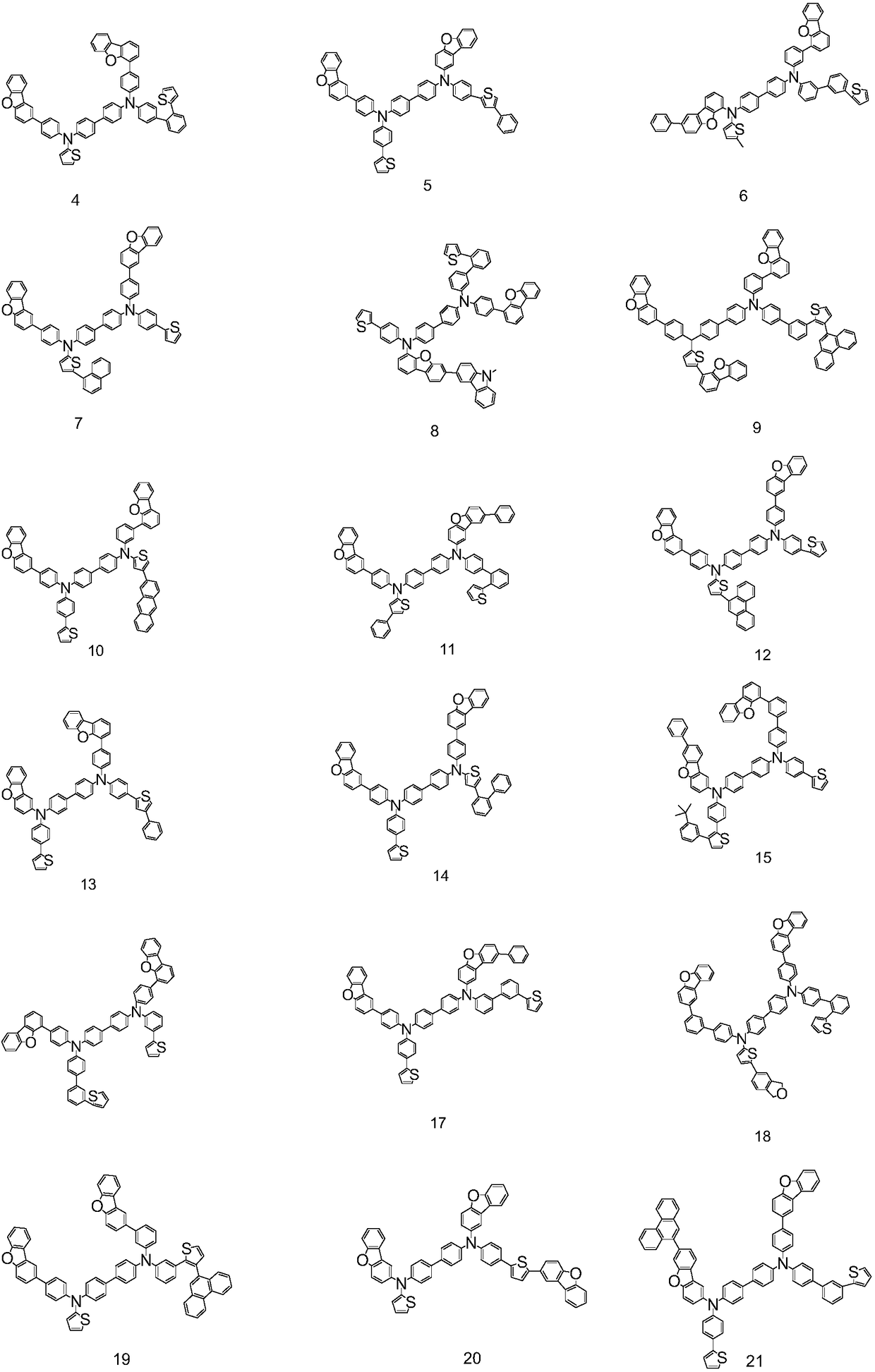
Organic electroluminescent compound, organic electroluminescence device and application thereof
A technology of luminescence and compounds, applied in the field of organic electroluminescence compounds, can solve the problems of high driving voltage, unsatisfactory luminous efficiency, and low luminous efficiency of light-emitting devices, and achieve low driving voltage, high luminous efficiency, and thermal stability good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of embodiment 1 compound (58)
[0051] (1) Synthesis of Intermediate-1
[0052] [Reaction 1]
[0053]
[0054] 20.4g (100mmol, 1.0eq.) 5-phenyl-2-thienyl boronic acid and 27.3g (110mmol, 1.1eq.) 4-bromo-4'-aminobiphenyl were added to a 2L three-necked flask, Add 1000ml of toluene and 100ml of ethanol to dissolve, pass nitrogen gas for 15 minutes, then add 150ml of 2M containing 41.5g (300mmol, 3.0eq.) K 2 CO 3 Aqueous solution, finally add 2.3g Pd(PPh 3 ) 4 (2mol%). The temperature was raised to 110° C., and the reaction was completed overnight. Add activated carbon for adsorption, filter with suction, spin off the solvent, dry, and recrystallize with toluene and ethanol to obtain 26.5 g of Intermediate-1 with a yield of 81%.
[0055] (2) Synthesis of Intermediate-2
[0056] [Reaction 2]
[0057]
[0058] Add 26.5g (81mmol, 1.1eq.) intermediate-1 and 23.8g (73.6mmol, 1.0eq.) 4-(4-bromophenyl)-dibenzo Furan, then add dry and degassed 1000ml ...
Embodiment 2
[0082] The synthesis of embodiment 2 compound (76)
[0083] (1) Synthesis of Intermediate-7
[0084] [Reaction 8]
[0085]
[0086] In a dry 2L three-necked flask, put 24.4g (100mmol, 1.0eq.) of 4-phenyldibenzofuran into it, dissolve it in 300ml of dry tetrahydrofuran, pass nitrogen gas, drop to -10°C, and add dropwise 44ml of 2.5M ( 110mmol, 1.1eq.) of LDA, after the dropwise addition, continue to stir at this temperature for 1 hour, add 38.1g (150mmol, 1.5eq.) of iodine in batches, remove the cooling environment, and continue to react overnight for 17 hours. At the end, add 4M HCl solution dropwise, stir for 1 hour, let stand, separate layers, wash the upper organic phase with water 3 times, extract the lower organic phase with dichloromethane, wash 3 times, combine the organic phases, dry, spin off the solvent, layer Purified by column analysis to obtain 32.6g of Intermediate-7 with a yield of 88%.
[0087] (2) Synthesis of Intermediate-8
[0088] [reaction formula 9...
Embodiment 3
[0122] The synthesis of embodiment 3 compound (99)
[0123] (1) Synthesis of Intermediate-15
[0124] [Equation 17]
[0125]
[0126]Add 17.2g (100mmol, 1.0eq.) of p-bromoaniline and 23.3g (110mmol, 1.1eq.) of dibenzofuran-4-boronic acid into a 2L three-necked flask, add 800ml of toluene and 80ml of ethanol to dissolve, and blow nitrogen 15 minutes, then add 150ml 2M containing 41.5g (300mmol, 3.0eq.) K 2 CO 3 Aqueous solution, finally add 2.3g Pd(PPh 3 ) 4 (2mol%). The temperature was raised to 100° C., and the reaction was completed overnight. Add activated carbon for adsorption, filter with suction, spin off the solvent, dry, and recrystallize with toluene and ethanol to obtain 21g of Intermediate-15 with a yield of 81%.
[0127] (2) Synthesis of Intermediate-16
[0128] [Equation 18]
[0129]
[0130] In a dry 2L three-necked flask, put 21g (81mmol, 1.0eq.) of the intermediate-15 obtained in Reaction Formula 17, dissolve it in 200ml of dry tetrahydrofuran, b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com