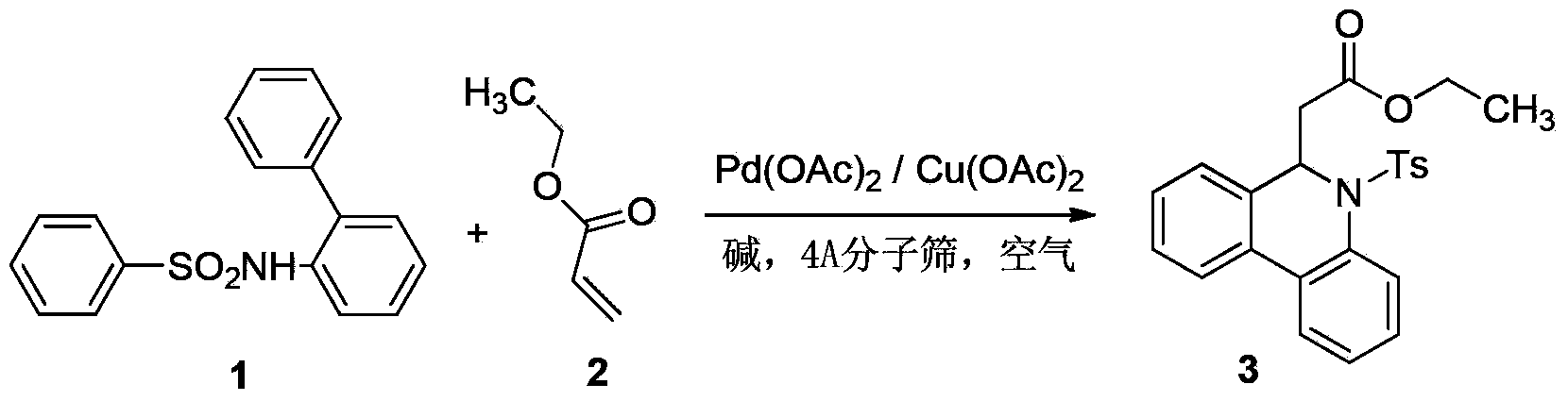
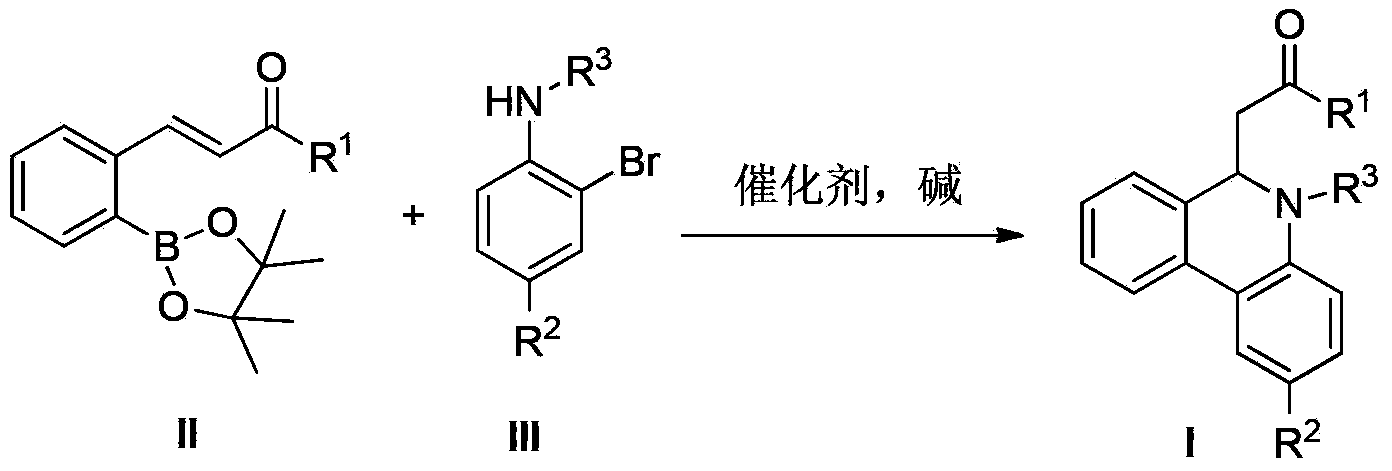
Preparation method of 6-substitute-5,6-dihydro phenanthridine derivative
A technology for dihydrophenanthridine and derivatives, which is applied in the field of preparation of 6-substituted-5,6-dihydrophenanthridine derivatives, can solve problems such as difficulty in the source of biphenyl derivatives 1, and achieve a wide range of substrate adaptation , high yield and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
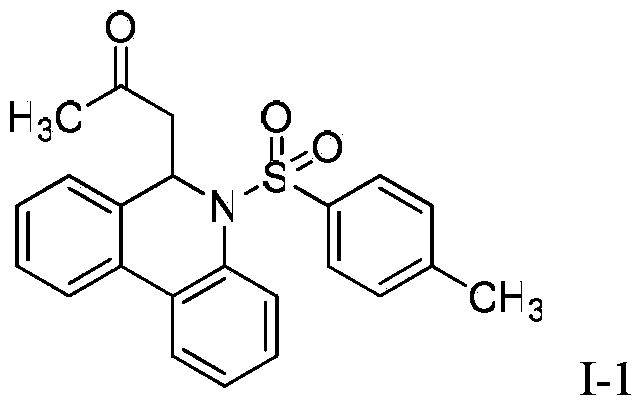
[0019] Synthesis of 6-(3-oxopropyl)-5-(4-toluenesulfonyl)-5,6-dihydrophenanthridine (I-1):
[0020]
[0021] In a 500ml three-necked bottle, (E)-2-(3-oxo-1-butenyl) phenylboronic acid pinacol ester (II-1: R=CH 3 ) (2.72g, 0.01mol), N-(4-toluenesulfonyl)-2-bromoaniline (3.24g, 0.01mol), tetrakis (triphenylphosphine) palladium (1.16g, 0.001mol) and 2mol / L Sodium carbonate aqueous solution (6.36g, 0.06mol) was completely dissolved in 300mL of ethylene glycol dimethyl ether, protected by nitrogen gas, heated to reflux for 6h, after the completion of the reaction monitored by TLC, cooled to room temperature, filtered, and the filtrate was concentrated under reduced pressure, the residue Separation by column chromatography gave yellow oily product I-1, weighing 2.76 g, with a yield of 84.9%.
[0022] 1 H-NMR (300MHz, CDCl3) δ7.74 (dd, J=7.6, 1.4Hz, 1H), 7.57 (dd, J=7.4, 1.7Hz, 1H), 7.43–7.29 (m, 2H), 7.23–7.13 (m,2H),7.13–7.02(m,2H),6.88(d,J=8.2Hz,2H),6.65(d J=8.1Hz,2H),5.82(t...
Embodiment 2
[0025] Synthesis of 2-methyl-6-(3-oxopropyl)-5-(4-toluenesulfonyl)-5,6-dihydrophenanthridine (I-2):
[0026] With (E)-2-(3-oxo-1-butenyl) phenylboronic acid pinacol ester (II-1: R=CH 3 ) and 4-methyl-N-(4-toluenesulfonyl)-2-bromoaniline as raw materials, and the operation was the same as in Example 1 to obtain yellow oily product I-2 with a yield of 92%.
[0027]
[0028] 1 H-NMR (300MHz, CDCl3) δ7.66(d, J=8.2Hz, 1H), 7.40(s, 1H), 7.26–7.17(m, 3H), 7.16–7.20(m, 2H), 6.94(d ,J=8.3Hz,2H),6.70(d,J=8.0Hz,2H),5.81(t,J=7.2Hz,1H),2.71(dd,J=16.2,7.4Hz,1H),2.50(dd ,J=16.1,7.2Hz,1H),2.44(s,3H),2.16(s,3H),2.14(s,3H).
[0029] 13 C-NMR (75MHz, CDCl 3 )δ204.61,142.32,137.00,133.89,133.52,130.03,129.37,129.16,129.02,128.75,127.81,127.26,127.04,126.78,126.60,126.45,123.61,122.62,122.02,54.95,48.37,29.88,20.91,20.72.
Embodiment 3
[0031] Synthesis of 2-fluoro-6-(3-oxopropyl)-5-(4-toluenesulfonyl)-5,6-dihydrophenanthridine (I-3):
[0032] With (E)-2-(3-oxo-1-butenyl) phenylboronic acid pinacol ester (II-1: R=CH 3 ) and 4-fluoro-N-(4-toluenesulfonyl)-2-bromoaniline as raw materials, and the operation was the same as in Example 1 to obtain yellow solid product I-3 with a yield of 83% and a melting range of 123-125°C.
[0033]
[0034] 1 H-NMR (300MHz, CDCl 3 )δ7.75(dd, J=8.9,5.3Hz,1H),7.26(dd,J=9.33,3.0Hz,1H),7.21(s,1H),7.19–7.06(m,4H),6.91(d ,J=8.3Hz,2H),6.69(d,J=8.0Hz,2H),5.84(t,J=7.2Hz,1H),2.68(dd,J=16.2,7.3Hz,1H),2.50(dd ,J=16.2,7.3Hz,1H),2.14(s,3H),2.12(s,3H).
[0035] 13 C-NMR (75MHz, CDCl 3 )δ204.10,163.02,159.84,142.70,133.94,133.12,131.42,131.31,130.90,130.77,128.15,127.95,127.28,126.59,122.88,115.28,115.01,109.82,109.51,54.70,48.37,29.31,20.82.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com