Glucose responsive gold nanoparticle and preparation method and application thereof
A technology of gold nanoparticles and glucose, which is applied in the fields of chemistry and material science, can solve problems such as interference of detection results and achieve the effect of avoiding interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
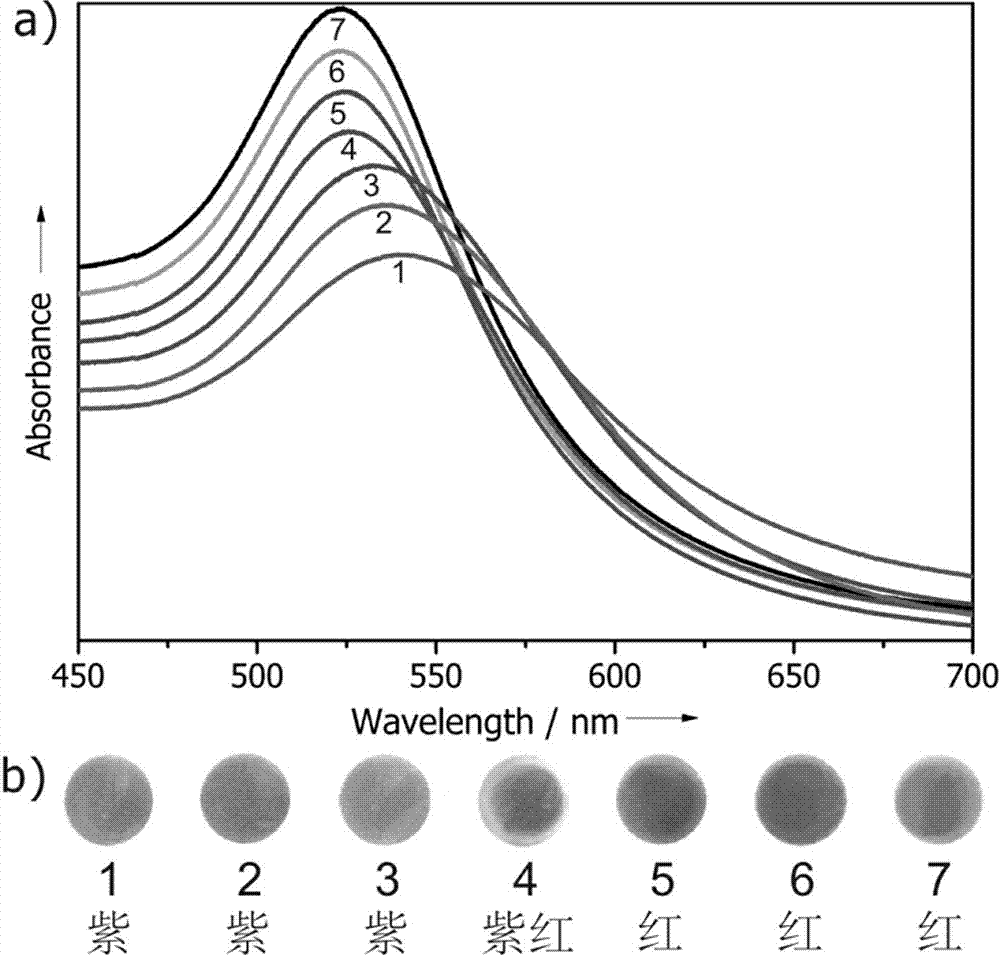
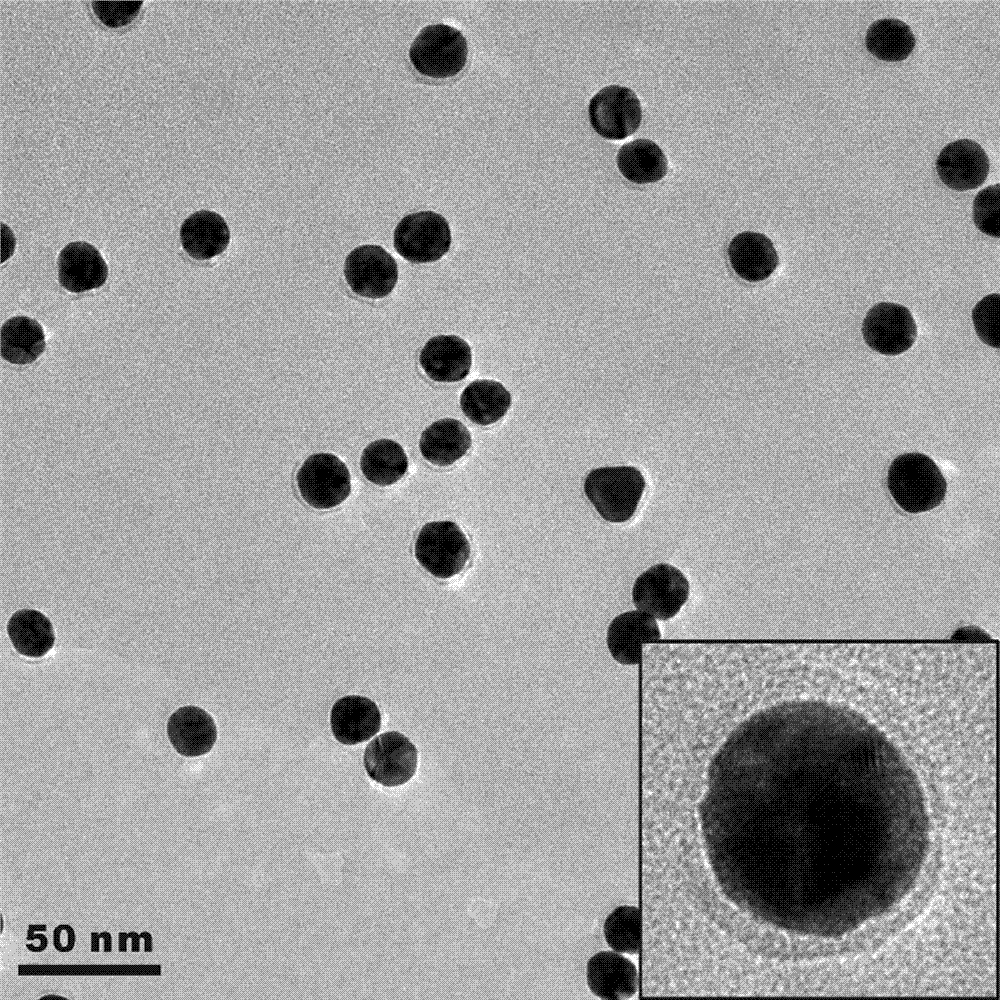
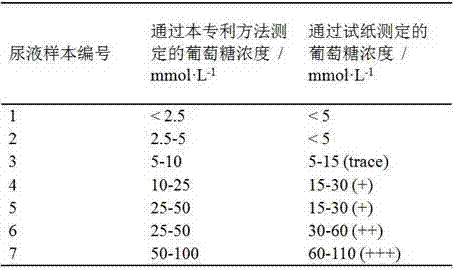
[0034] This example describes the method in detail by modifying gold nanoparticles with isopropylacrylamide-acryloylthioureidoaminophenylboronic acid copolymer and demonstrating its response process to glucose molecules:
[0035] (1) Synthesis of gold nanoparticles (AuNPs): 1 g of chloroauric acid powder was dissolved in 100 mL of ultrapure water to obtain a 1% chloroauric acid stock solution. Add 1 mL of chloroauric acid stock solution to 100 mL of ultrapure water, stir and heat to boiling. 1 mL of trisodium citrate solution (1 mmol) was quickly added, and the solution turned red after a few minutes. Continue heating and stirring for about 15 minutes, cool to room temperature, and store at 4°C for later use to obtain a solution of gold nanoparticles coated with sodium citrate.
[0036] (2) Synthesis of acryloylthioureidoaminophenylboronic acid: Weigh 0.727 g of potassium thiocyanate, dissolve it in 15 mL of anhydrous acetone, add 0.678 g of acryloyl chloride (7.5 mmol), and ...
Embodiment 2
[0049] This example describes the method in detail by modifying gold nanoparticles with isopropylacrylamide-acryloylthioureidoaminophenylboronic acid copolymer and demonstrating its response process to glucose molecules:
[0050] (1) Synthesis of gold nanoparticles (AuNPs): 1 g of chloroauric acid powder was dissolved in 100 mL of ultrapure water to obtain a 1% chloroauric acid stock solution. Add 1 mL of chloroauric acid stock solution to 100 mL of ultrapure water, stir and heat to boiling. 1 mL of trisodium citrate solution (1 mmol) was quickly added, and the solution turned red after a few minutes. Continue heating and stirring for about 15 minutes, cool to room temperature, and store at 4°C for later use to obtain a solution of gold nanoparticles coated with sodium citrate.
[0051] (2) Synthesis of acryloylthioureidoaminophenylboronic acid: Weigh 0.727 g of potassium thiocyanate, dissolve it in 15 mL of anhydrous acetone, add 0.678 g of acryloyl chloride (7.5 mmol), and ...
Embodiment 3
[0057] (1) Synthesis of gold nanoparticles (AuNPs): 1 g of chloroauric acid powder was dissolved in 100 mL of ultrapure water to obtain a 1% chloroauric acid stock solution. Add 1 mL of chloroauric acid stock solution to 100 mL of ultrapure water, stir and heat to boiling. 1 mL of trisodium citrate solution (1 mmol) was quickly added, and the solution turned red after a few minutes. Continue heating and stirring for about 15 minutes, cool to room temperature, and store at 4°C for later use to obtain a solution of gold nanoparticles coated with sodium citrate.
[0058] (2) Synthesis of acryloylthioureidoaminophenylboronic acid: Weigh 0.727 g of potassium thiocyanate, dissolve it in 15 mL of anhydrous acetone, add 0.678 g of acryloyl chloride (7.5 mmol), and stir overnight at room temperature. The reaction solution was filtered, and the yellow supernatant was taken for later use. Weigh 1.0 g of 3-aminophenyl borate hydrochloride (5.77 mmol) and dissolve it in 40 mL of acetone / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com