Phenanthridine compound and synthesis method thereof
A synthesis method and compound technology, applied in organic chemistry and other fields, can solve the problems of low functional group tolerance, few reports, high reaction cost, etc., and achieve the effect of cheap reaction raw materials, easy operation, and lower reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of Ethylphenanthridine-6-carboxylate
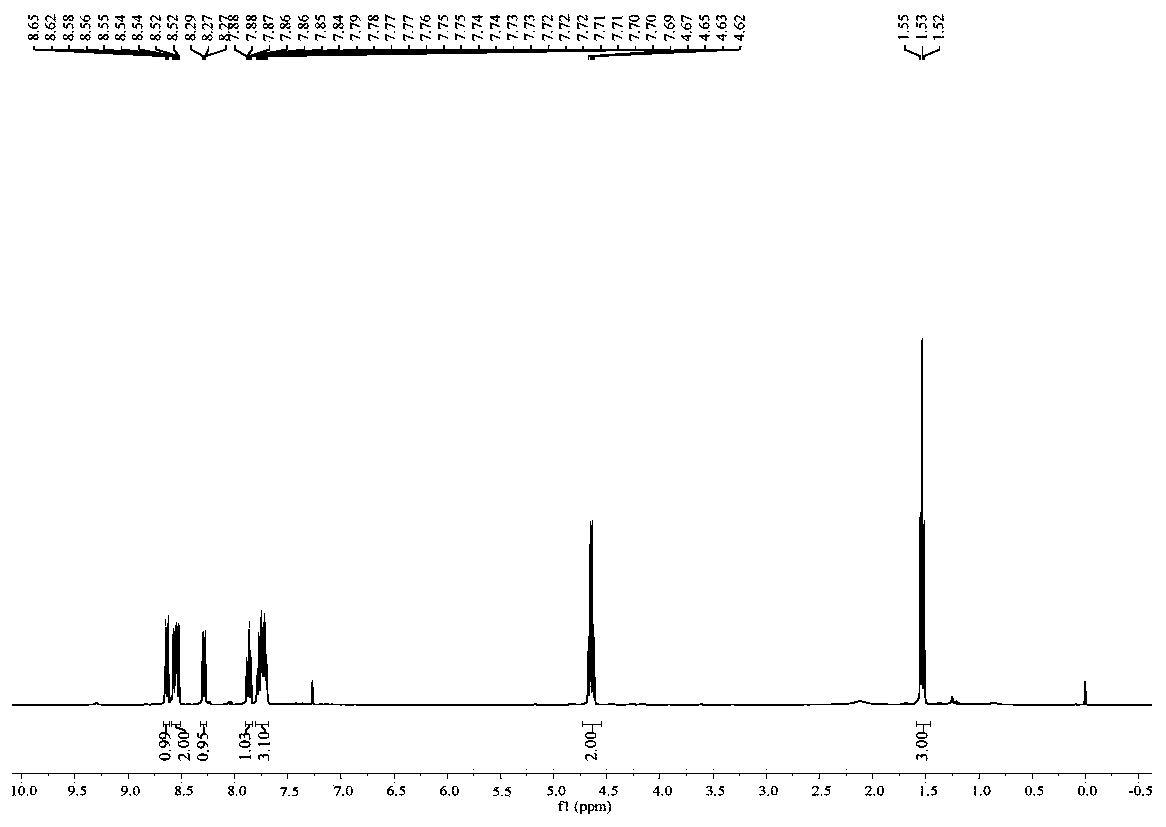
[0065] 0.2 mmol of 1,1'-diphenyl-2-amine, 1.5 equivalents of ethyl 2-diazobenzoylacetate and 1.6 mL of CF 3 Add COOH to a 15mL pressure-resistant tube (equipped with a magnetic stirrer), stir at 120°C under oxygen, monitor the progress of the reaction with TLC, and extract three times with 15mL ethyl acetate and saturated 15*3mL sodium bicarbonate solution after the reaction is complete , the combined organic phases were concentrated and separated by silica gel column chromatography to obtain 38.2 mg of light yellow solid compound with a yield of 76%. The structural formula of the resulting product is as follows:
[0066]
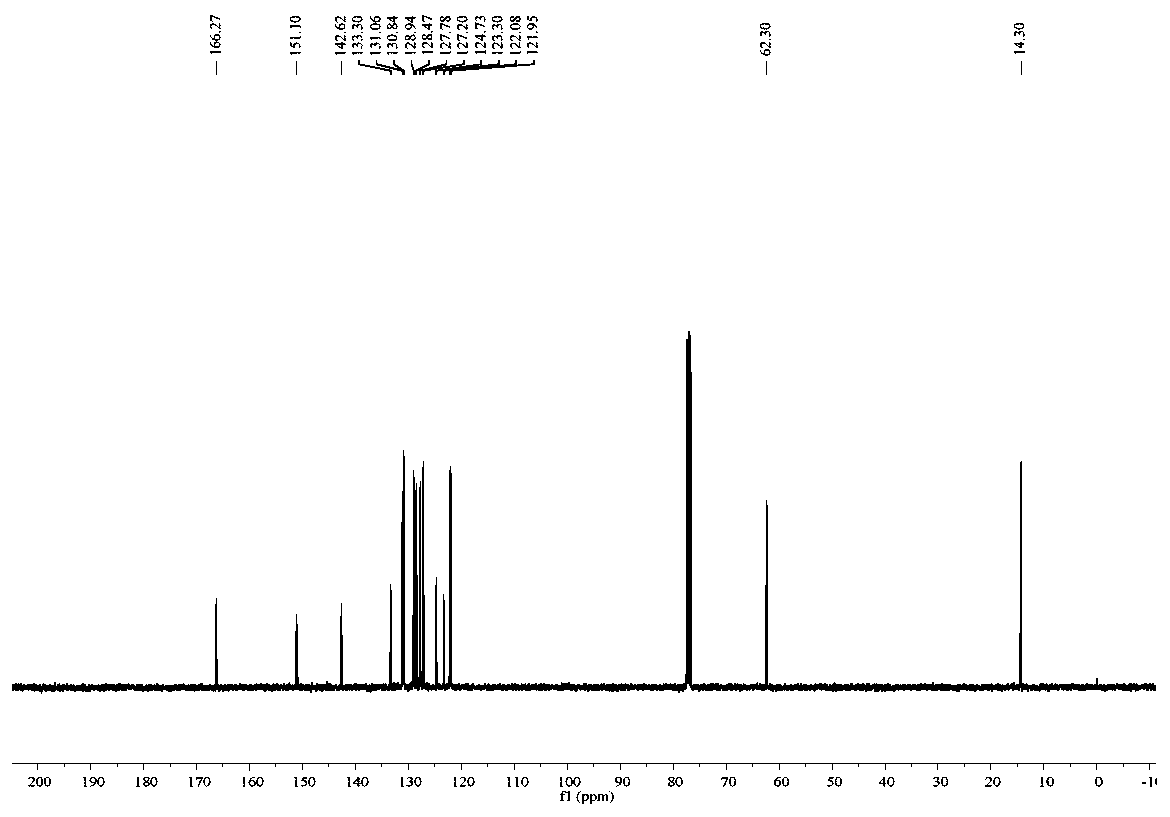
[0067] like figure 1 and figure 2 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 ):δ8.64(d,J=8.3Hz,1H),8.59-8.51(m,2H),8.29(d,J=7.6Hz,1H),7.86(t,J=7.2Hz,1H),7.80 -7.67(m,3H),4.64(q,J=7.1Hz,2H),1.53(t,J=7.1Hz,3H). 13 C NMR (100MHz, CDCl 3 ): δ166.3, 151.1, 142.6, 133.3, 131.1,...
Embodiment 2
[0070] Preparation of Ethyl 8-methylphenanthridine-6-carboxylate
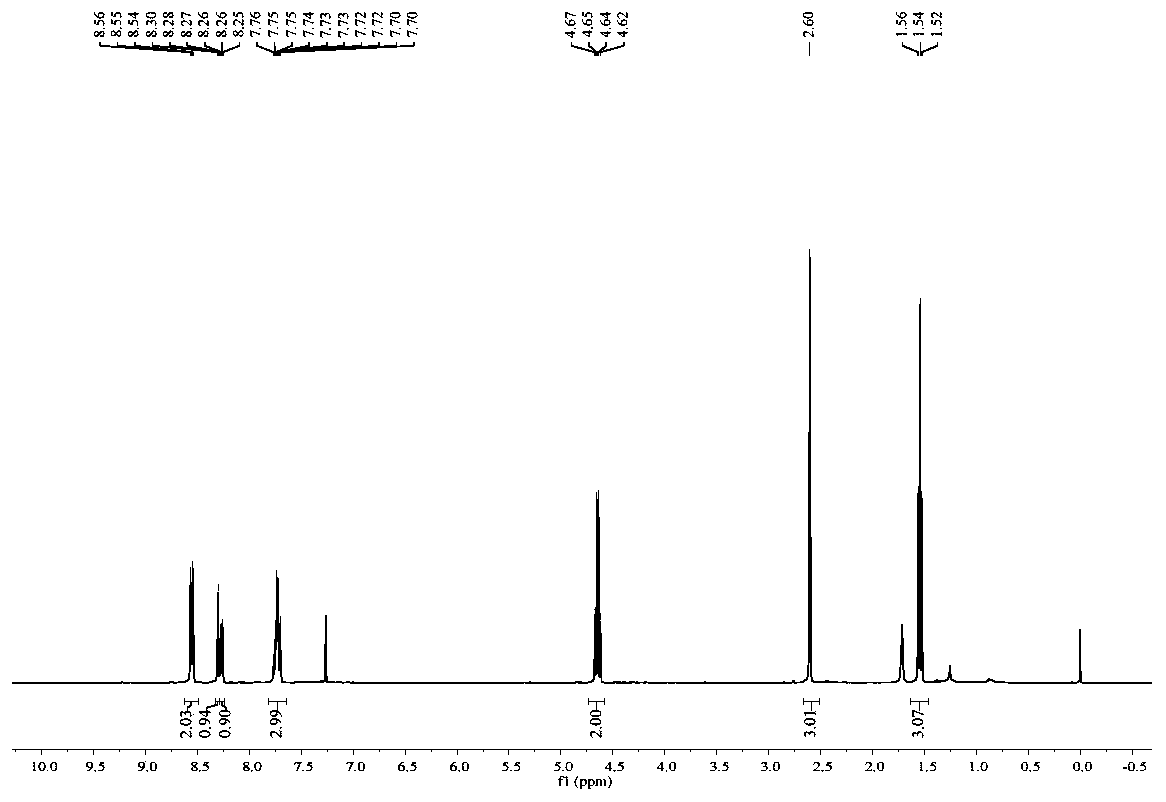
[0071] Mix 0.2 mmol of 4'-methyl-1,1'-diphenyl-2-amine, 1.5 equivalents of ethyl 2-diazobenzoylacetate and 1.6 mL of CF 3 Add COOH to a 15mL pressure-resistant tube (equipped with a magnetic stirrer), stir at 120°C under oxygen, monitor the progress of the reaction with TLC, and extract three times with 15mL ethyl acetate and saturated 15*3mL sodium bicarbonate solution after the reaction is complete , the combined organic phases were concentrated and separated by silica gel column chromatography to obtain 38.2 mg of a white solid compound with a yield of 72%. The structural formula of the resulting product is as follows:
[0072]
[0073] like image 3 and Figure 4 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 ): δ8.55(d, J=8.5Hz, 2H), 8.30(s, 1H), 8.29-8.24(m, 1H), 7.82-7.64(m, 3H), 4.64(q, J=7.1Hz, 2H), 2.60(s, 3H), 1.54(t, J=7.1Hz, 3H). 13 C NMR (100MHz, CDCl 3 ): δ166.4, 150.8, 14...
Embodiment 3
[0076] Preparation of Ethyl 8-methoxyphenanthridine-6-carboxylate
[0077] Mix 0.2 mmol of 4'-methoxy-1,1'-diphenyl-2-amine, 1.5 equivalents of ethyl 2-diazobenzoylacetate and 1.6 mL of CF 3 Add COOH to a 15mL pressure-resistant tube (equipped with a magnetic stirrer), stir at 120°C under oxygen, monitor the progress of the reaction with TLC, and extract three times with 15mL ethyl acetate and saturated 15*3mL sodium bicarbonate solution after the reaction is complete , the combined organic phases were concentrated and separated by silica gel column chromatography to obtain 37.7 mg of a white solid compound with a yield of 67%. The structural formula of the resulting product is as follows:
[0078]
[0079] like Figure 5 and Image 6 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 ):δ8.56(d,J=9.1Hz,1H),8.51-8.47(m,1H),8.29-8.24(m,1H),8.03(d,J=2.6Hz,1H),7.73-7.70( m,2H),7.50(dd,J=9.1,2.6Hz,1H),4.64(q,J=7.1Hz,2H),3.99(s,3H),1.55(t,J=7.1Hz,3H). 13 C NMR (10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com