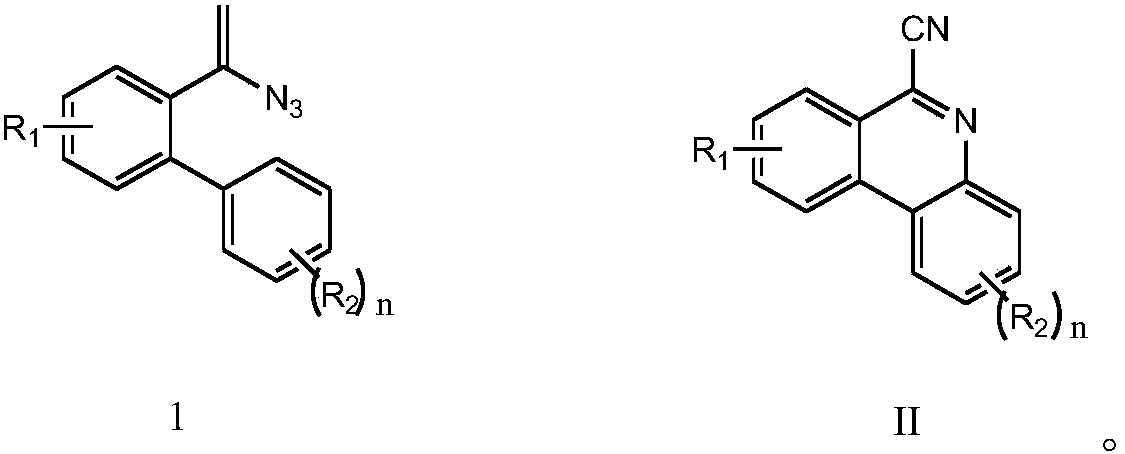
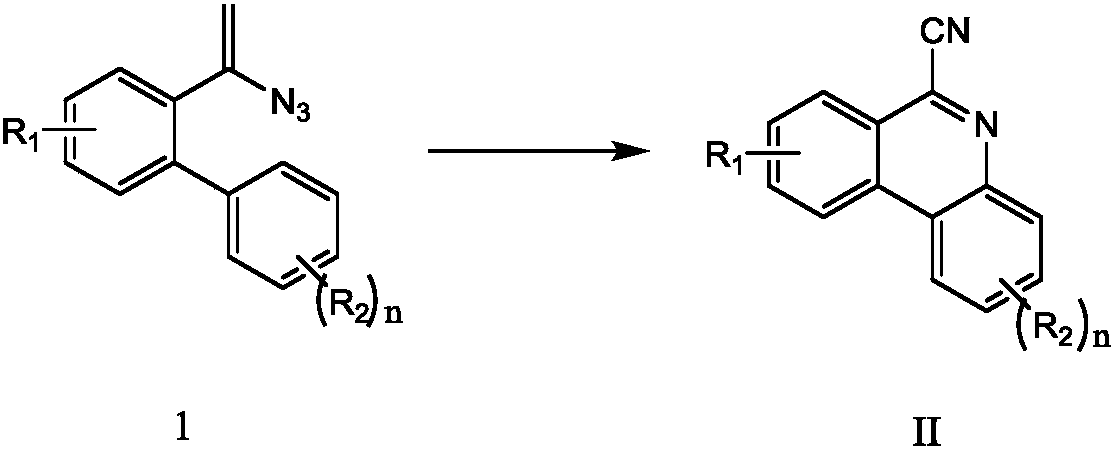
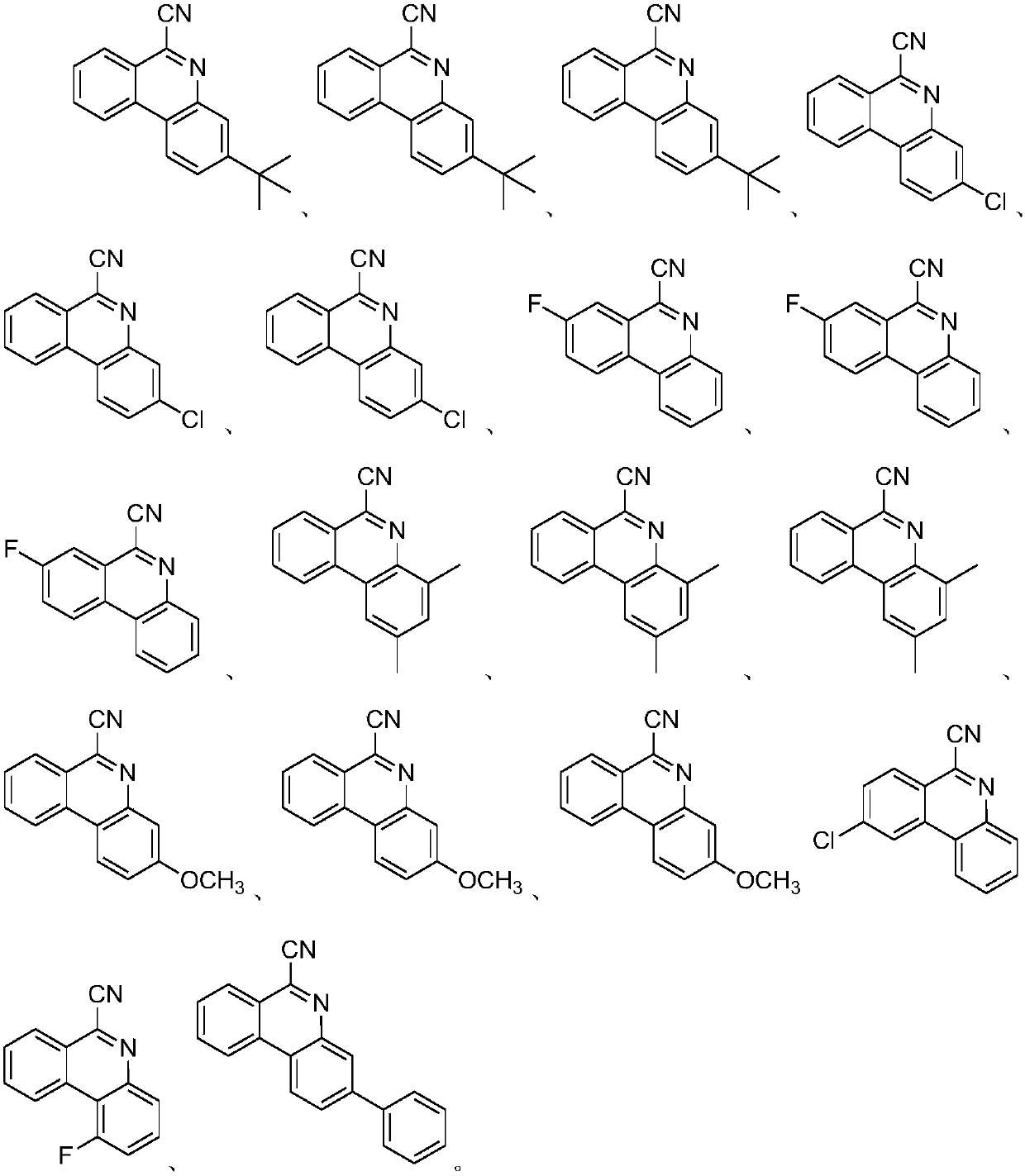
Method for synthesizing 6-cyano phenanthridine compound
A technology of cyanophenanthridine and compounds, which is applied in the field of synthesis of organic compounds, can solve problems such as complex catalytic systems and high reaction temperatures, and achieve the effects of simple reaction steps, mild reaction conditions, and good substrate adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] Add 0.3 mmol of 2-(4-tert-butyl)phenyl-α-azido-styrene, 0.3 mmol of sodium azide, 0.6 mmol of iodobenzene diacetate, and 0.03 mmol of copper sulfate to a 15 mL thick-wall pressure-resistant reaction In the tube, add 3 mL of acetonitrile as solvent. Next, it was magnetically stirred at 40° C. for 4 hours. After cooling to room temperature, two spoons of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then the pure product was obtained by column chromatography (as petroleum ether / ethyl acetate = 50:1 as eluent). The material was an off-white solid in 55% yield.
[0027] Characterization data: 1 H NMR (500MHz, CDCl 3 ): δ8.63(d, J=8.0Hz, 1H), 8.53(d, J=8.5Hz, 1H), 8.42(d, J=8.0Hz, 1H), 8.24(d, J=1.5Hz, 1H ),7.98–7.89(m,2H),7.84–7.78(m,1H),1.49(s,9H); 13C NMR(125MHz,CDCl3)δ153.3,143.7,135.5,132.6,132.1,128.4,128.4,126.7, 126.6, 125.2, 122.4, 122.2, ...
Embodiment 2
[0029]
[0030] Add 0.3 mmol of 2-(4-tert-butyl)phenyl-α-azido-styrene, 0.6 mmol of sodium azide, 0.6 mmol of iodobenzene diacetate, and 0.03 mmol of copper powder to a 15 mL thick-wall pressure-resistant reaction In the tube, 3 mL of tetrahydrofuran was added as a solvent. Next, stir magnetically at 40° C. for 4 hours. After cooling to room temperature, two spoons of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then the pure product was obtained by column chromatography (as petroleum ether / ethyl acetate = 50:1 as eluent). The material was an off-white solid in 50% yield.
[0031] Characterization data: 1 H NMR (500MHz, CDCl 3 ): δ8.63(d, J=8.0Hz, 1H), 8.53(d, J=8.5Hz, 1H), 8.42(d, J=8.0Hz, 1H), 8.24(d, J=1.5Hz, 1H ),7.98–7.89(m,2H),7.84–7.78(m,1H),1.49(s,9H); 13C NMR(125MHz,CDCl3)δ153.3,143.7,135.5,132.6,132.1,128.4,128.4,126.7, 126.6, 125.2, 122.4, 122.2, ...
Embodiment 3
[0033]
[0034] Add 0.3 mmol of 2-(4-tert-butyl)phenyl-α-azido-styrene, 0.3 mmol of sodium azide, 0.6 mmol of iodobenzene diacetate, and 0.03 mmol of copper acetate to 15 mL of thick-wall pressure-resistant reaction In the tube, 3 mL of tetrahydrofuran was added as a solvent. Next, stir magnetically at 40° C. for 4 hours. After cooling to room temperature, two spoons of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and then the pure product was obtained by column chromatography (as petroleum ether / ethyl acetate = 50:1 as eluent). The material was an off-white solid, 53% yield.
[0035] Characterization data: 1 H NMR (500MHz, CDCl 3 ): δ8.63(d, J=8.0Hz, 1H), 8.53(d, J=8.5Hz, 1H), 8.42(d, J=8.0Hz, 1H), 8.24(d, J=1.5Hz, 1H ),7.98–7.89(m,2H),7.84–7.78(m,1H),1.49(s,9H); 13C NMR(125MHz,CDCl3)δ153.3,143.7,135.5,132.6,132.1,128.4,128.4,126.7, 126.6, 125.2, 122.4, 122.2, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com