Phenanthridine derivative as well as medicinal composition, preparation method and application thereof
The technology of a compound and general formula is applied in the application field of preparing anti-hepatitis C and hepatitis B virus drugs, and can solve the problems of no phenanthridine derivatives, no anti-hepatitis B and anti-hepatitis C activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
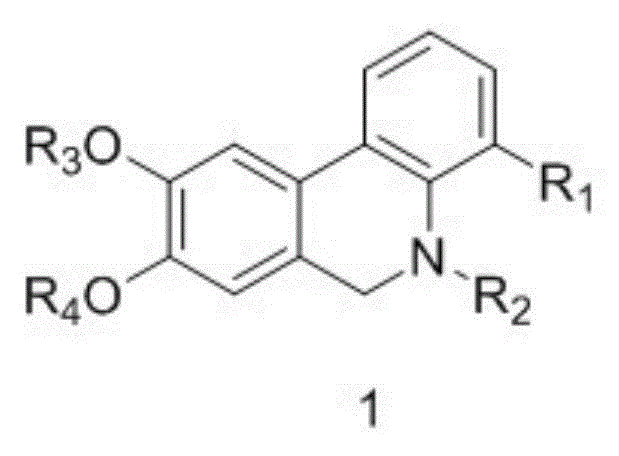
[0043] Synthesis of compounds of general formula (1):
[0044] (1) Experimental conditions.
[0045] ESI and high-resolution mass spectrometry were determined by Finnigan MAT 90 and VG Auto Spec-3000 mass spectrometer; melting point was determined by X-4 melting point instrument (Yuhua Experimental Equipment Factory, Gongyi, Henan); nuclear magnetic spectrum was determined by Bruker AM-400, DRX- 500 and Avance III 600 NMR analyzers, deuterated chloroform and deuterated DMSO as solvents, Me 4 Si as internal standard; Silica gel: 60-80 mesh, 300-400 mesh (Shandong Qingdao Ocean Chemical Company); Silica gel plate: Pre-coated silica gel 60 F 254 (Merck, Darmstadt, Germany); HPLC: Hypersil Gold RP-C 18 column (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA); reagents, solvents: Aldrich-sigma Chemical Co., Acros Organics and J&K Scientific.
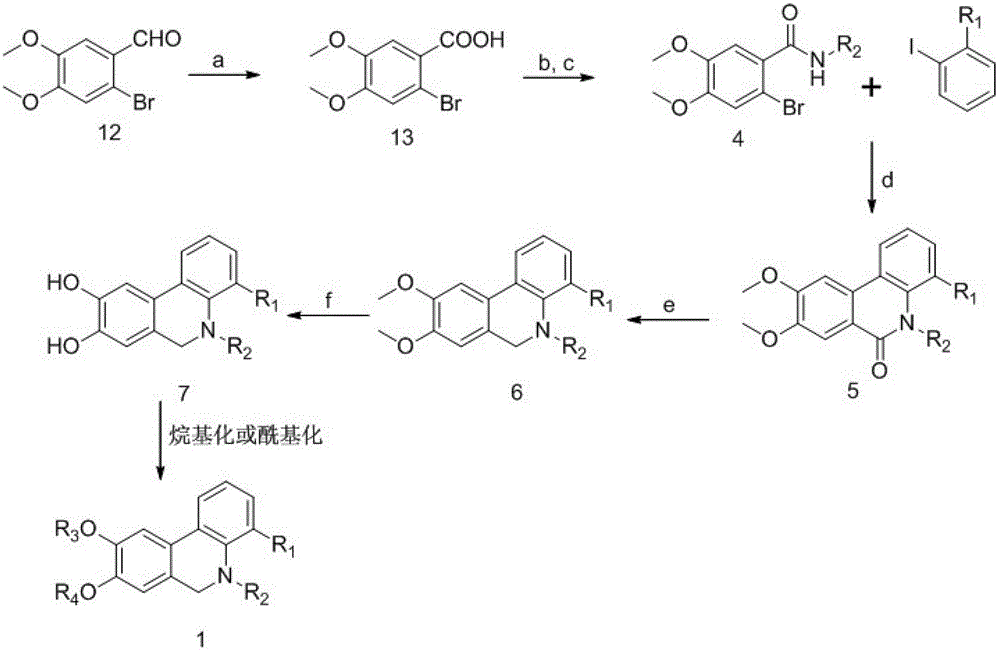
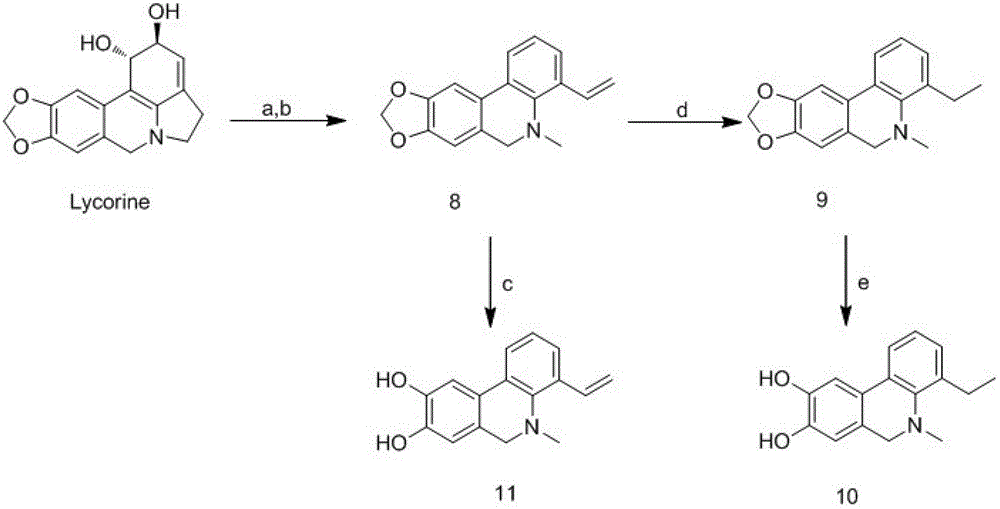
[0046] (2) Total synthesis of the compound of general formula (1):
[0047]
[0048] Reagents and conditions: a. NaHCO ...
Embodiment 2
[0054] Synthesis of Specific Compounds:
[0055] (1) 2-bromo-4,5-dimethoxybenzoic acid (13): 2-bromo-4,5-dimethoxybenzaldehyde (250mg, 1mmol), sodium bicarbonate (200mg) and potassium permanganate ( 500 mg) was dissolved in water (20 mL), heated and stirred for 3 hours, then extracted twice with 20 mL of dichloromethane, combined organic phases, washed with saturated ammonium chloride solution and saturated brine, and dried overnight with anhydrous magnesium sulfate, After filtration, the excess solvent was evaporated, and the residue was purified by silica gel column chromatography to obtain compound 13 (220 mg, yield: 85%) as a light yellow solid.
[0056] (2) 2-bromo-4,5-dimethoxy-N-methyl-Benzenemethanamine (14): Dissolve compound 13 (260 mg, 1 mmol) in THF (10 mL), then add DMF (0.1 mL) and thionyl chloride (0.5mL, 4mmol), the reaction solution was stirred at 50°C for 2 hours, then the excess THF was evaporated under reduced pressure, and the residue was added dropwise t...
Embodiment 3
[0106] Anti-hepatitis C virus (HCV) activity of the compounds of the present invention:
[0107] (1) Experimental method
[0108] (1) Toxicity test of compounds on Huh7.5 cells:
[0109] 1×10 5 / mL Huh7.5 cells were seeded in 100 μL in a 96-well plate, at 37 °C, 5 % CO 2 After culturing in an incubator under saturated humidity conditions for 24 hrs, different concentrations of the compound solution of the present invention and positive control drug (VX-950) were added, and after continuing to culture for 72 hrs, 10 μL of 5 mg / mL was added to each well MTT, continue to culture for 4 hrs, after lysing with DMSO, measure OD on a microplate reader 570-630 mm Compared with the OD value of the cell control group, the toxicity inhibition rate of each concentration to the cells was calculated, and the half toxic concentration of the drug to the cells was calculated by the Reed-Muench method.
[0110] (2) Anti-HCV activity of the compound in cell culture:
[0111] 1×10 5 / mL Huh7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com